Unit dose flexible container
a flexible container and unit dose technology, applied in the field of liquid containers, can solve the problems of difficult development of economic packaging for unit doses, and affecting patient's ability to measure an accurate dos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
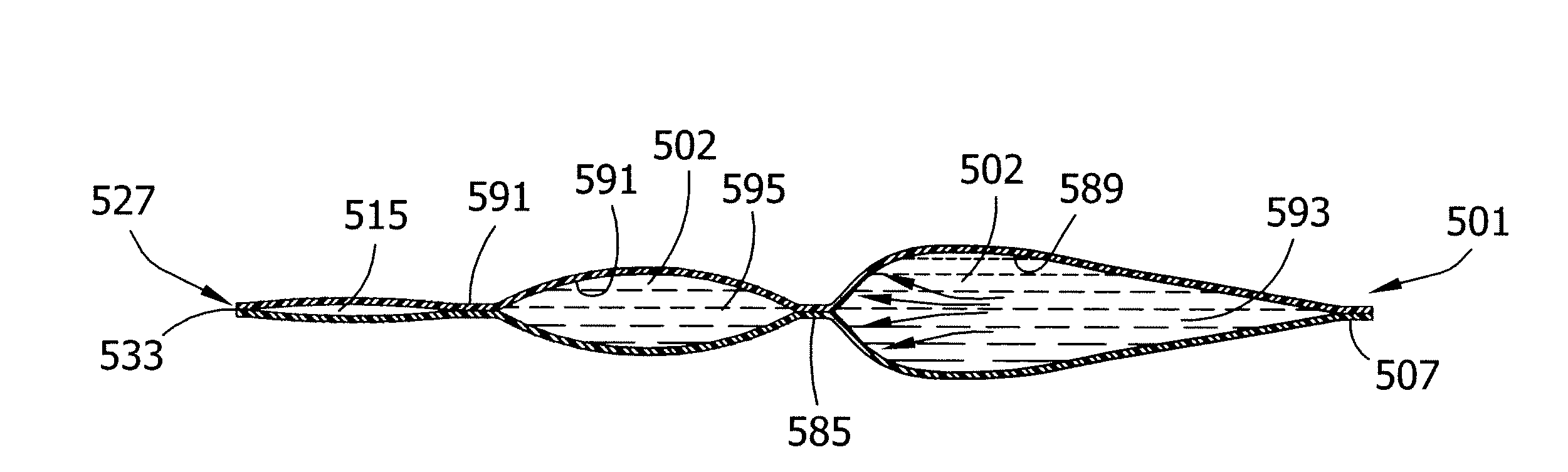
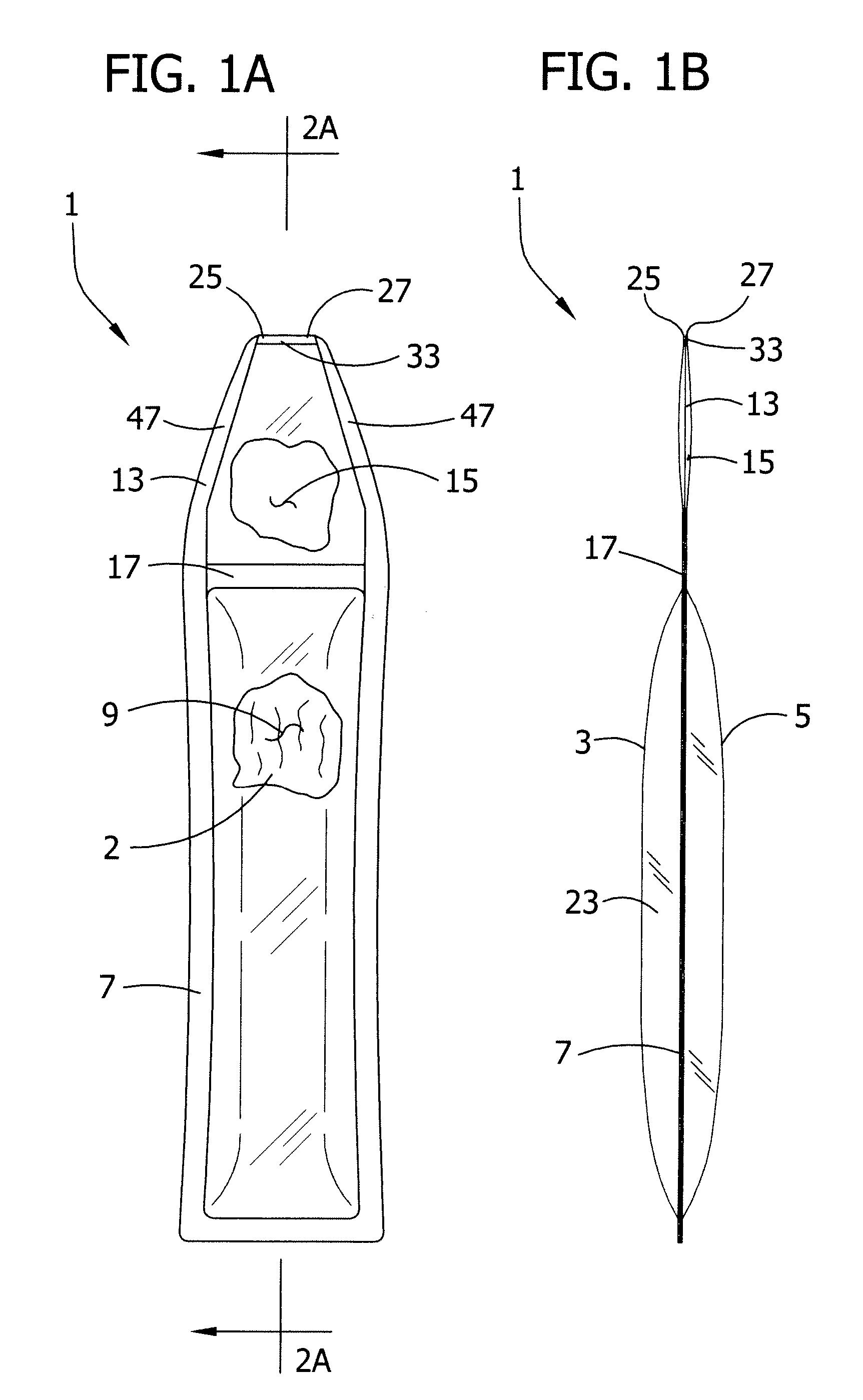
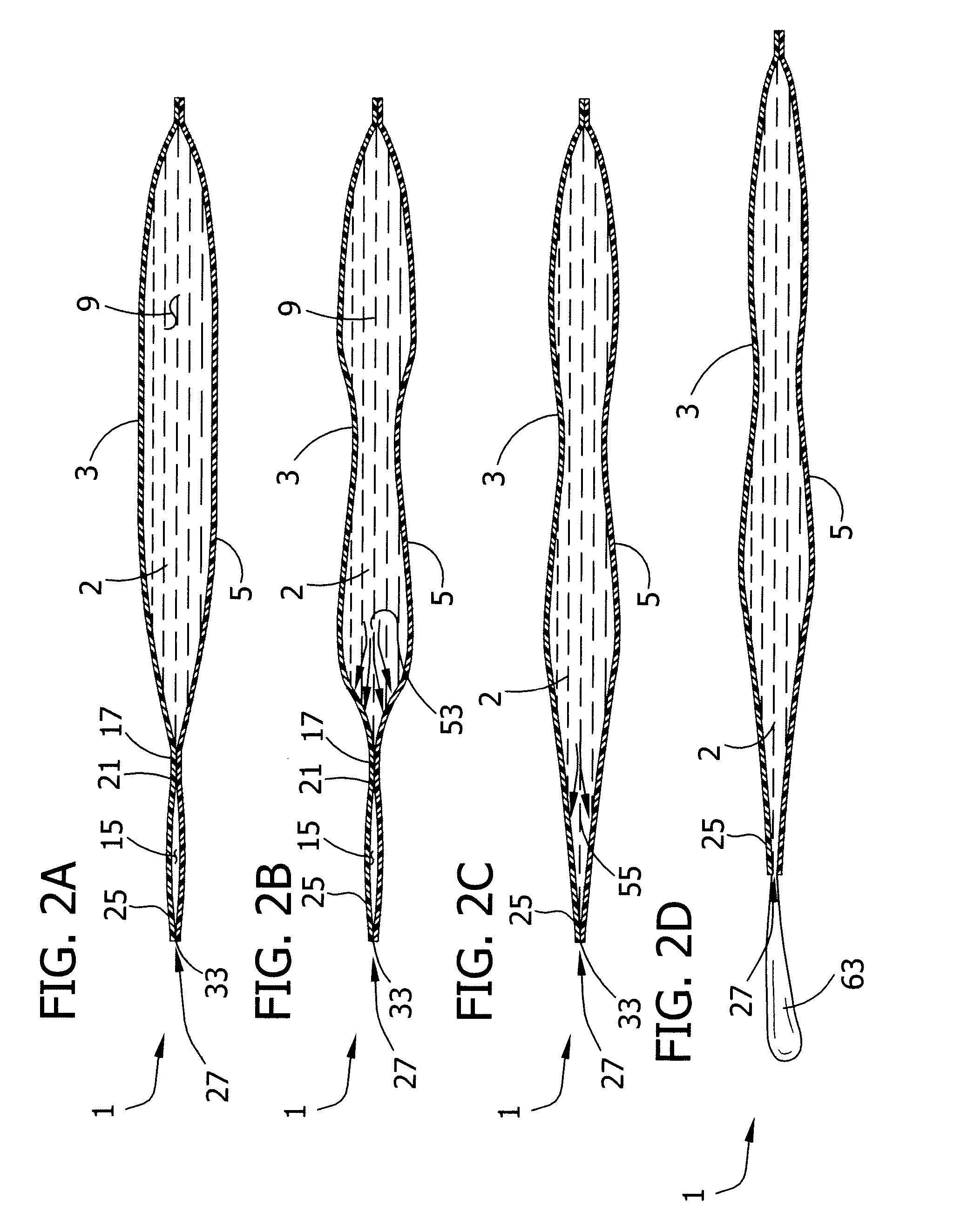
[0046] Referring to FIGS. 1A and 1B, for example, a first embodiment of a single use pliable container (generally indicated at 1) includes a single dose volume of liquid 2. The container further comprises first and second generally opposed pliable sheets designated at 3 and 5, respectively. A first seal 7 extends around a lower perimeter of the sheets 3, 5 and joins the sheets to partially define a liquid-containing chamber 9. A second seal 13 extends along most of an upper perimeter of the sheets 3, 5 and joins the sheets to partially define a pressure relieving chamber 15 adjacent the liquid-containing chamber 9. A boundary seal 17 joins the first and second sheets 3, 5 between the liquid-containing chamber 9 and the pressure relieving chamber 15. In the embodiment shown in FIGS. 1A and 1B the entire boundary seal 17 constitutes a fluid transfer region seal. However, it will be understood that the extent of the fluid transfer region seal may be limited to only a portion of the bou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosities | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com