Synergistic activation of regulatory elements by rel proteins and a steroid receptor
a technology of steroid receptor and receptor, which is applied in the direction of drug compositions, peptide sources, instruments, etc., can solve the problems of hampered development of compounds that exclusively target one set of tissues or organs (e.g. brain for psychiatric illnesses)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods Used
[0053] Special Reagents. 17β-estradiol was obtained from Sigma Chemical Co (St Louis, Mo.). 4-hydroxytamoxifen and ICI 164384 were kind gifts from Dr. A. Wakeling, Zeneca Pharmaceuticals, UK.
[0054] Cell Culture. Monkey COS-1 cells and human 293 embryonal kidney cells were obtained from American Type Culture Collection (ATCC; Rockville, MD) and were cultured in a 1:1 mixture of DMEM and Ham's F-12 medium (DF; Life Technologies Inc.), buffered with bicarbonate and supplemented with 7.5% FCS. Dextran-coated charcoal(DCC)-FCS was prepared by treatment of FCS with dextran-coated charcoal to remove steroids, as described previously (25).
[0055] Plasmids. −901 luc was created by partial digestion of −1588luc, a kind gift from Dr. O. Meijer (Leiden, the Netherlands), with Styl, filling-in and ligation into pGL3 digested with SmaI, redigestion with HindIII and religation; −81luc was created by digestion of −1588luc with StyI, filling-in and digestion with BglII an...
example 2
Synergistic Activation of the 5-HT1A Receptor Promoter by NF-κB and ER
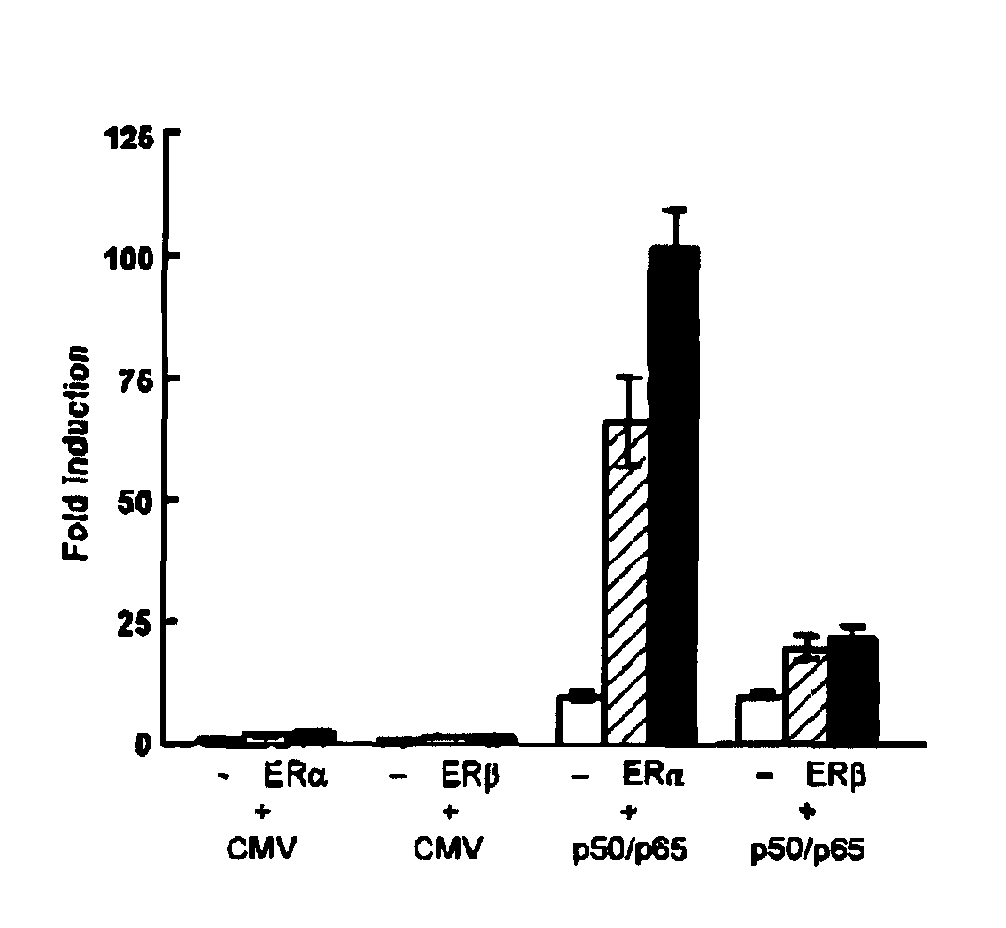
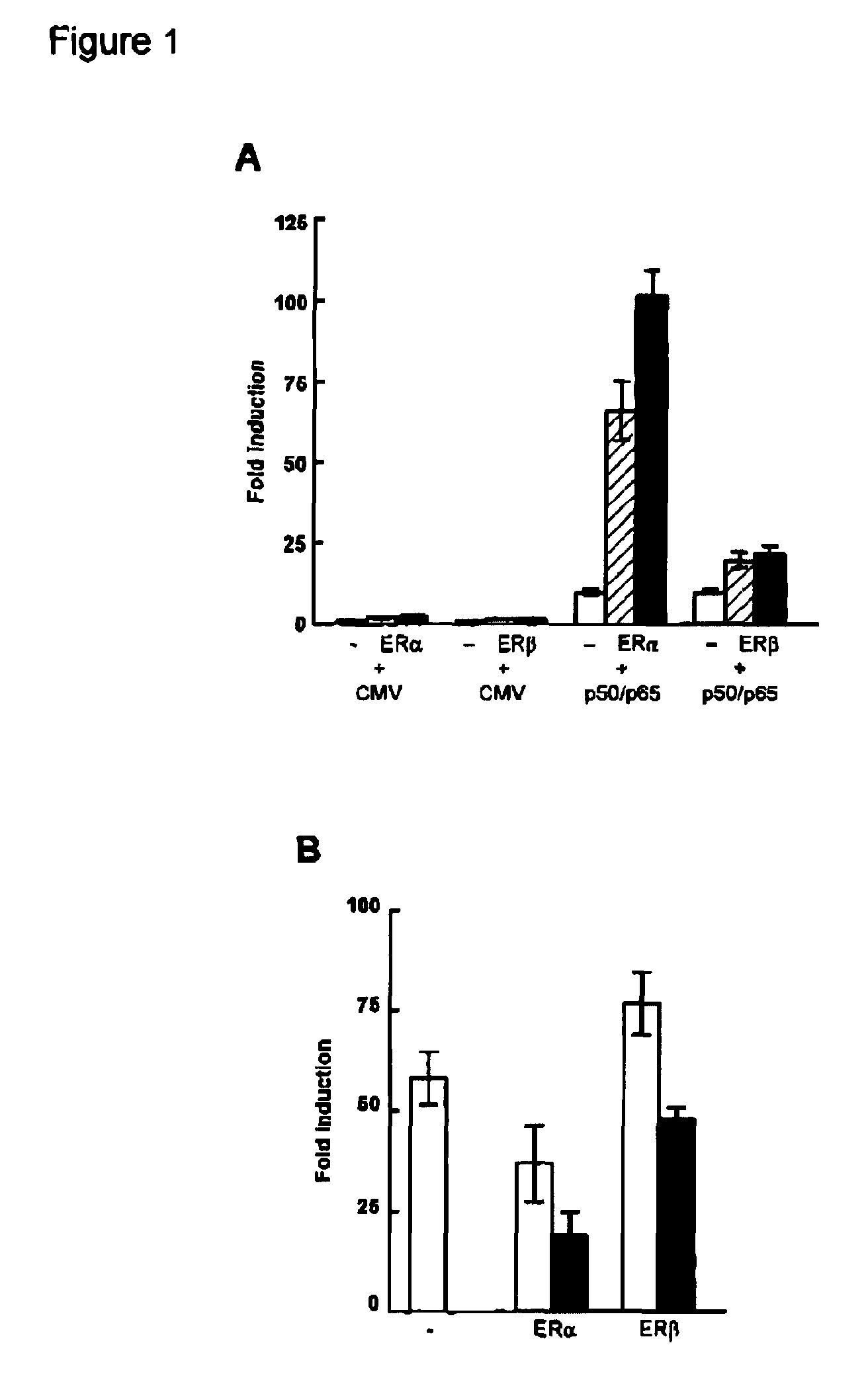
[0057] To determine the effect of estrogens on 5-HT1A receptor promoter activity, we transiently transfected COS-1 cells with a reporter construct containing the 5-HT1A receptor promoter together with an expression vector encoding ERα or ERβ. As shown in FIG. 1A, cotransfection of ERα or ERβ and treatment of the cells with 17β-estradiol (E2) hardly had an effect on 5-HT1A promoter activity. However, besides direct regulation, ER target genes can also be regulated indirectly through interaction of ER with other transcription factors. Putative NF-κB binding sites were shown to be present in the −901luc 5-HT1A receptor promoter construct (see FIG. 2A) and transfection of this reporter construct with expression vectors encoding the p50 and p65 subunit of NF-κB resulted in an 10-fold induction of the reporter. Interestingly, cotransfection of ERα in combination with NF-κB now resulted in an enormous induction of promo...
example 3
Involvement of NF-κB Elements in 5-HT1A Receptor Promoter Regulation by NF-κB and ER
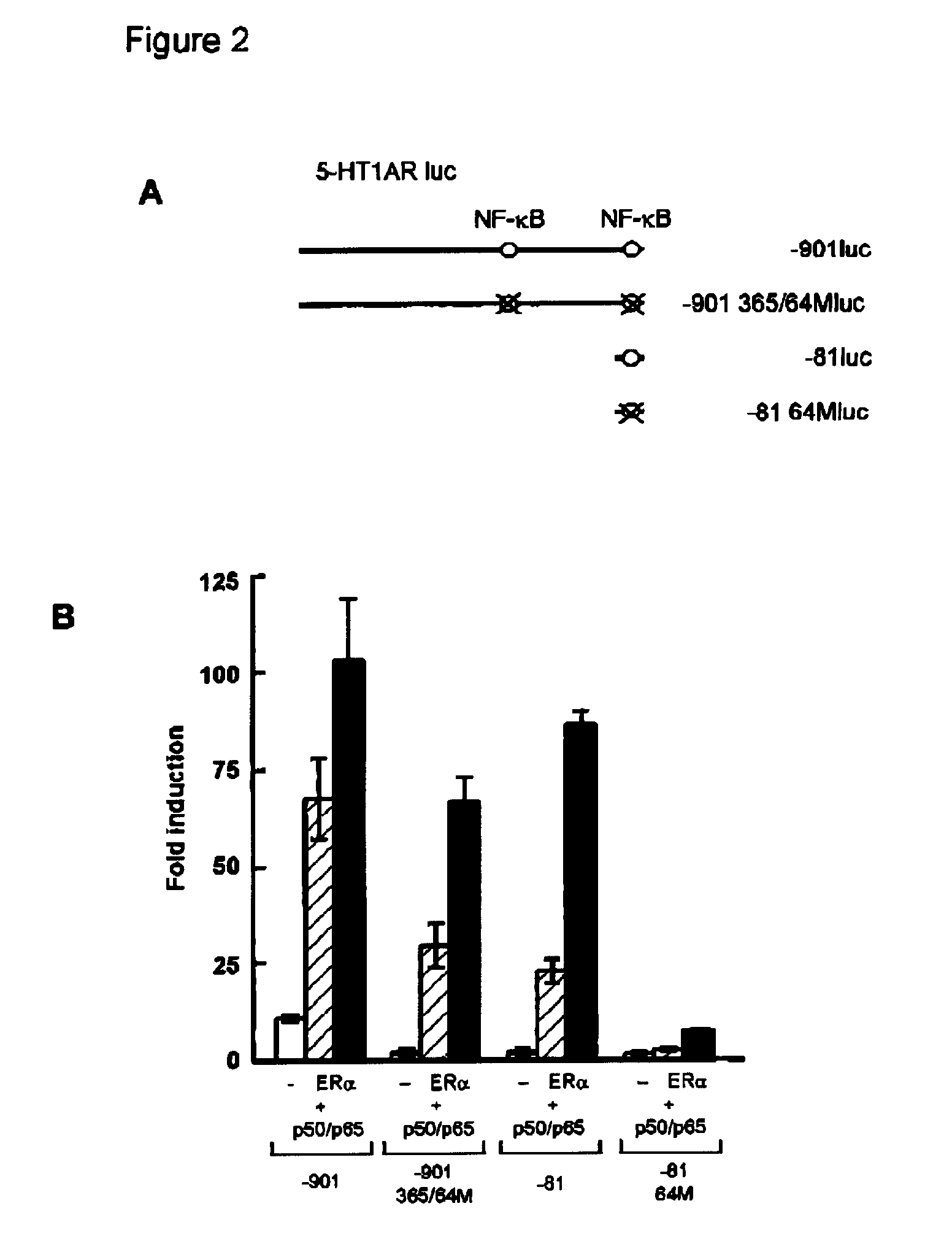
[0059] To localize the effect of ER on the 5-HT1A receptor promoter, several promoter deletion constructs were used (FIG. 2A). Mutation of both NF-κB elements (−901 365 / 64Mluc) completely abolished the effect of NF-κB on the 5-HT1A receptor promoter (FIG. 2B). However, ERα, only in combination with NF-κB, was still able to induce promoter activity as efficient as on the wild type promoter (−901 luc). Likewise, the promoter construct −81luc could not be induced by NF-κB, although it still contained one NF-κB element. However, also on this promoter construct, the effect of ERα with NF-κB was maintained. When the single NF-κB element present in the −81luc construct was mutated (−81 64Mluc), the ERα effect was almost completely abolished. Thus, synergistic activation of the 5-HT1A receptor promoter involves NF-κB binding sites, although activation of the promoter by NF-κB itself appears not to be requir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| modular structure | aaaaa | aaaaa |
| neuronal plasticity | aaaaa | aaaaa |
| Topcount liquid scintillation counter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com