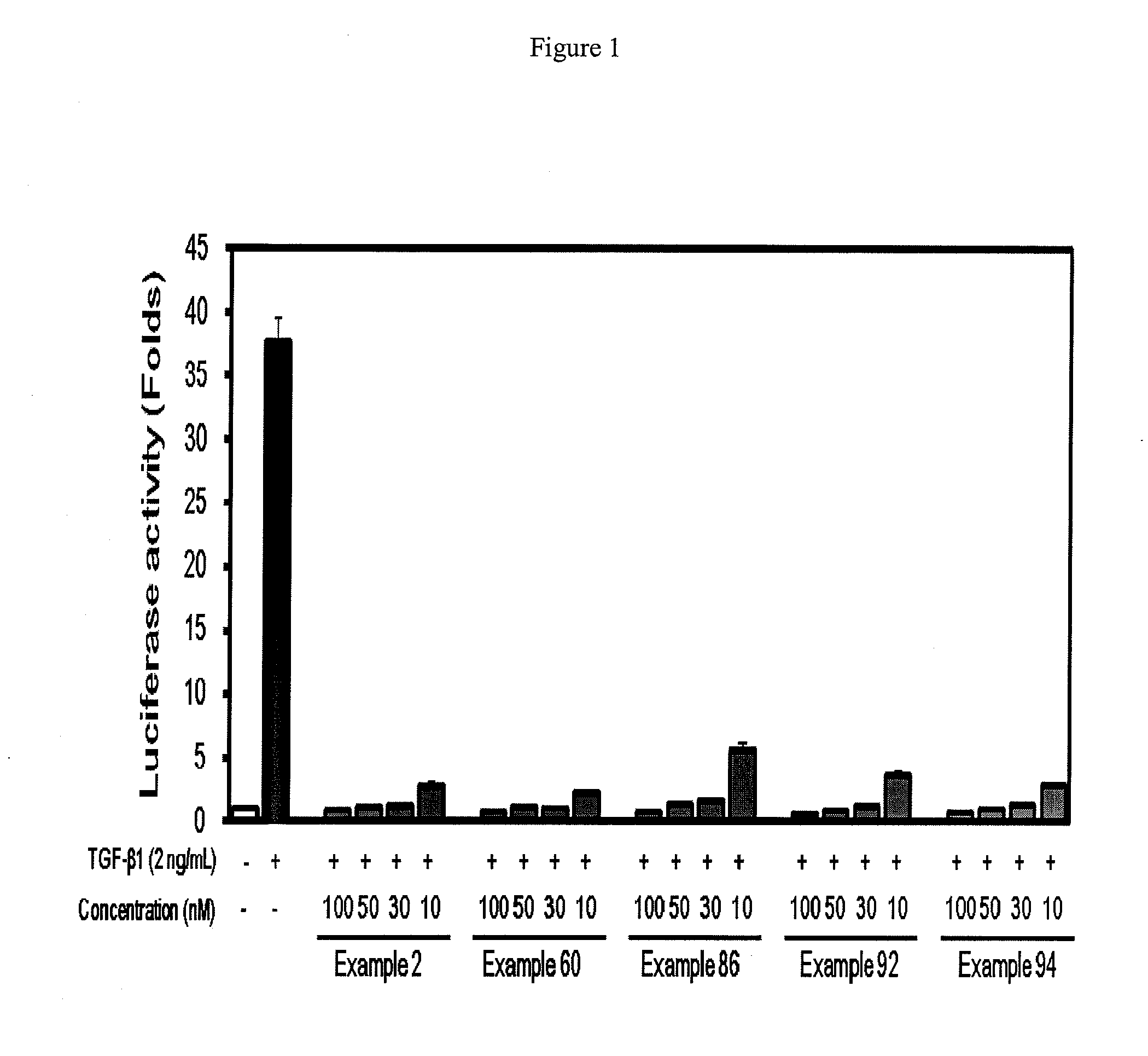
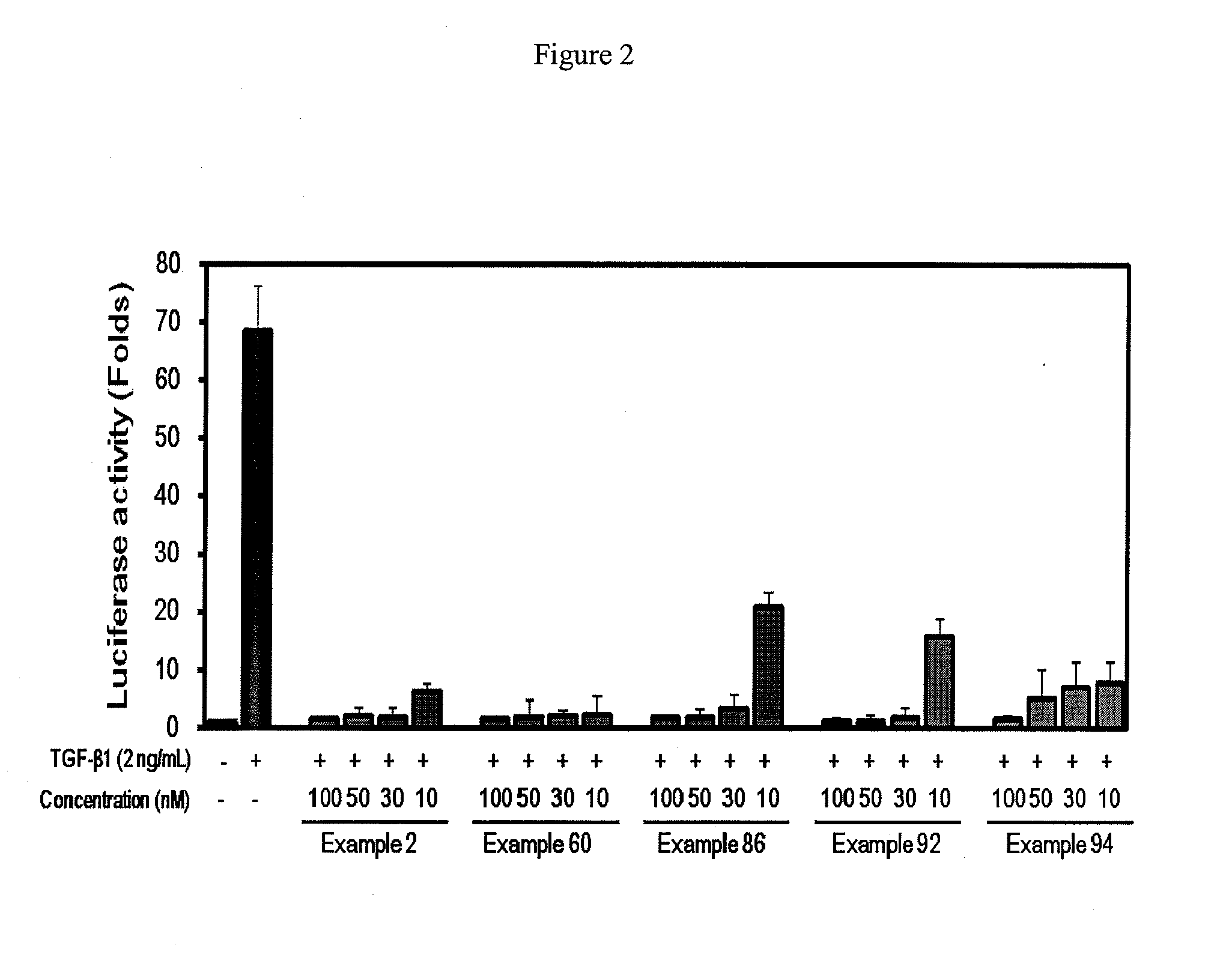
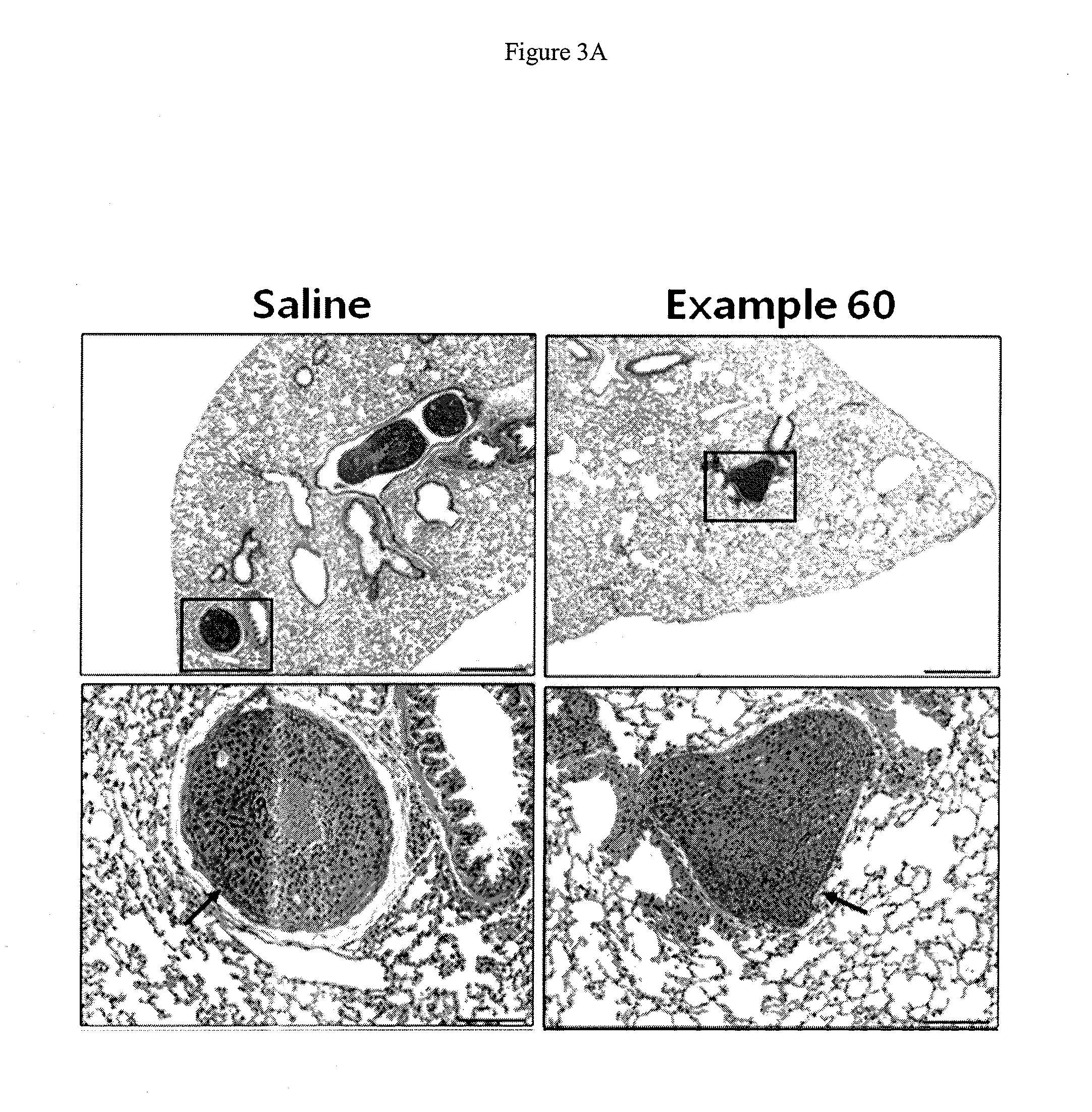
2-pyridyl substituted imidazoles as therapeutic alk5 and/or alk4 inhibitors
a technology of imidazoles and pyridyl substitutes, which is applied in the direction of drug compositions, metabolism disorders, extracellular fluid disorders, etc., and can solve problems such as complicated organ transplantation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 8
Preparative Example 8
Preparation of 6-(2-(dimethoxymethyl)-5-(6-ethylpyridin-2-yl)-1H-imidazol-4-yl)-[1,2,4]triazolo[1,5-a]pyridine (a Compound of the Formula (VI) wherein Ra═CH2CH3)
[0194]The titled compound was prepared as described in Preparative Example 7 by using 1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-ethylpyridin-2-yl)ethane-1,2-dione in place of 1-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-2-(6-methylpyridin-2-yl)ethane-1,2-dione. Yield: 68%; 1H NMR (400 MHz, CDCl3): δ 10.67 (br s, 1H), 8.97 (br s, 1H), 8.35 (s, 1H), 7.83 (dd, 1H, J=9.2, 1.6 Hz), 7.76 (dd, 1H, J=9.2, 0.8 Hz), 7.50 (t, 1H, J=7.8 Hz), 7.25 (br d, 1H, J=7.6 Hz), 7.05 (d, 1H, J=8.0 Hz), 5.56 (s, 1H), 3.46 (s, 6H), 2.83 (q, 2H, J=7.6 Hz), 1.31 (t, 3H, J=7.6 Hz).
example 9
Preparative Example 9
Preparation of 4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-methylpyridin-2-yl)-1H-imidazole-2-carbaldehyde (a Compound of the Formula (VII) wherein Ra═CH3)
[0195]6-(2-(Dimethoxymethyl)-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)-[1,2,4]triazolo[1,5-a]pyridine (6.00 g, 17.12 mmol) was dissolved in 1 N HCl (120 mL), and the mixture was heated at 70° C. for 3 h. The reaction mixture was allowed to cool to 0° C., and then it was neutralized with saturated aqueous NaHCO3 solution. The mixture was extracted with 10% MeOH in CHCl3 (3×200 mL), and the organic phase was dried over anhydrous Na2SO4, filtered, and evaporated to dryness under reduced pressure to give the titled compound (4.69 g, 90%) as a light yellow solid. 1H NMR (400 MHz, CDCl3): δ 9.82 (s, 1H), 9.01 (br s, 1H), 8.41 (s, 1H), 7.85 (dd, 1H, J=9.2, 0.8 Hz), 7.82 (dd, 1H, J=9.2, 1.6 Hz), 7.55 (t, 1H, J=7.8 Hz), 7.33 (br s, 1H), 7.16 (d, 1H, J=8. 0 Hz), 2.60 (s, 3H).
example 10
Preparative Example 10
Preparation of 4-([1,2,4]triazolo[1,5-a]pyridin-6-yl)-5-(6-ethylpyridin-2-yl)-1H-imidazole-2-carbaldehyde (a Compound of the Formula (VII) wherein Ra═CH2CH3)
[0196]The titled compound was prepared as described in Preparative Example 9 by using 6-(2-(dimethoxymethyl)-5-(6-ethylpyridin-2-yl)-1H-imidazol-4-yl)-[1,2,4]triazolo[1,5-a]pyridine in place of 6-(2-(dimethoxymethyl)-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)-[1,2,4]triazolo[1,5-a]pyridine. Yield: 99%; 1H NMR (400 MHz, DMSO-d6) δ 9.86 (t, 1H, J=1.2 Hz), 9.59 (s, 1H), 8.43 (s, 1H), 8.21 (dd, 1H, J=9.2, 1.6 Hz), 7.82 (br d, 1H, J=8.0 Hz), 7.73 (dd, 1H, J=9.2, 0.8 Hz), 7.69 (t, 1H, J=7.8 Hz), 7.08 (br d, 1H, J=7.6 Hz), 2.71 (q, 2H, J=7.6 Hz), 1.16 (t, 3H, J=7.6 Hz).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com