Treatment of multiple sclerosis (MS)
a technology for multiple sclerosis and treatment, applied in the field of treatment of multiple sclerosis, to achieve the effect of significant efficacy and favorable safety profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
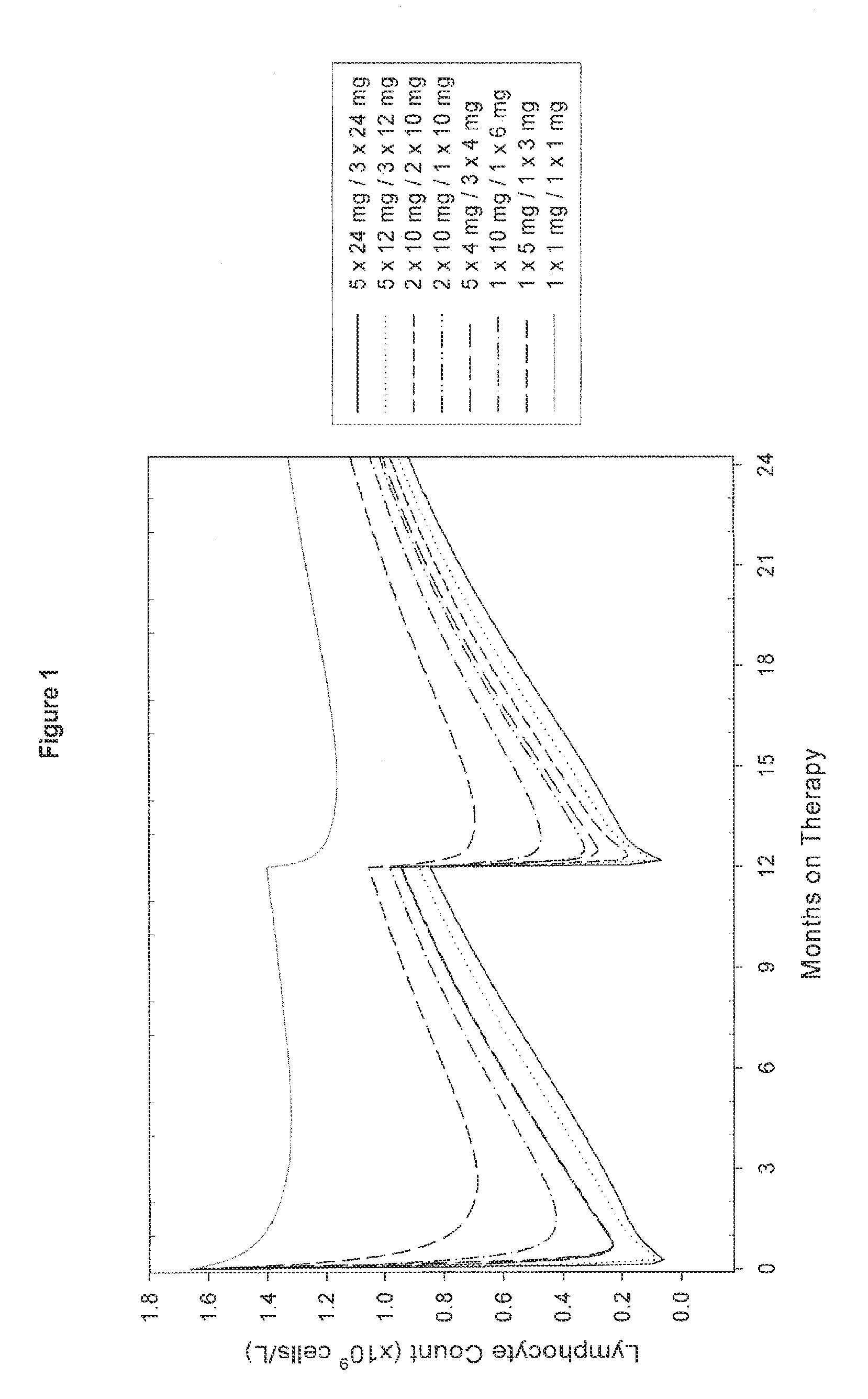
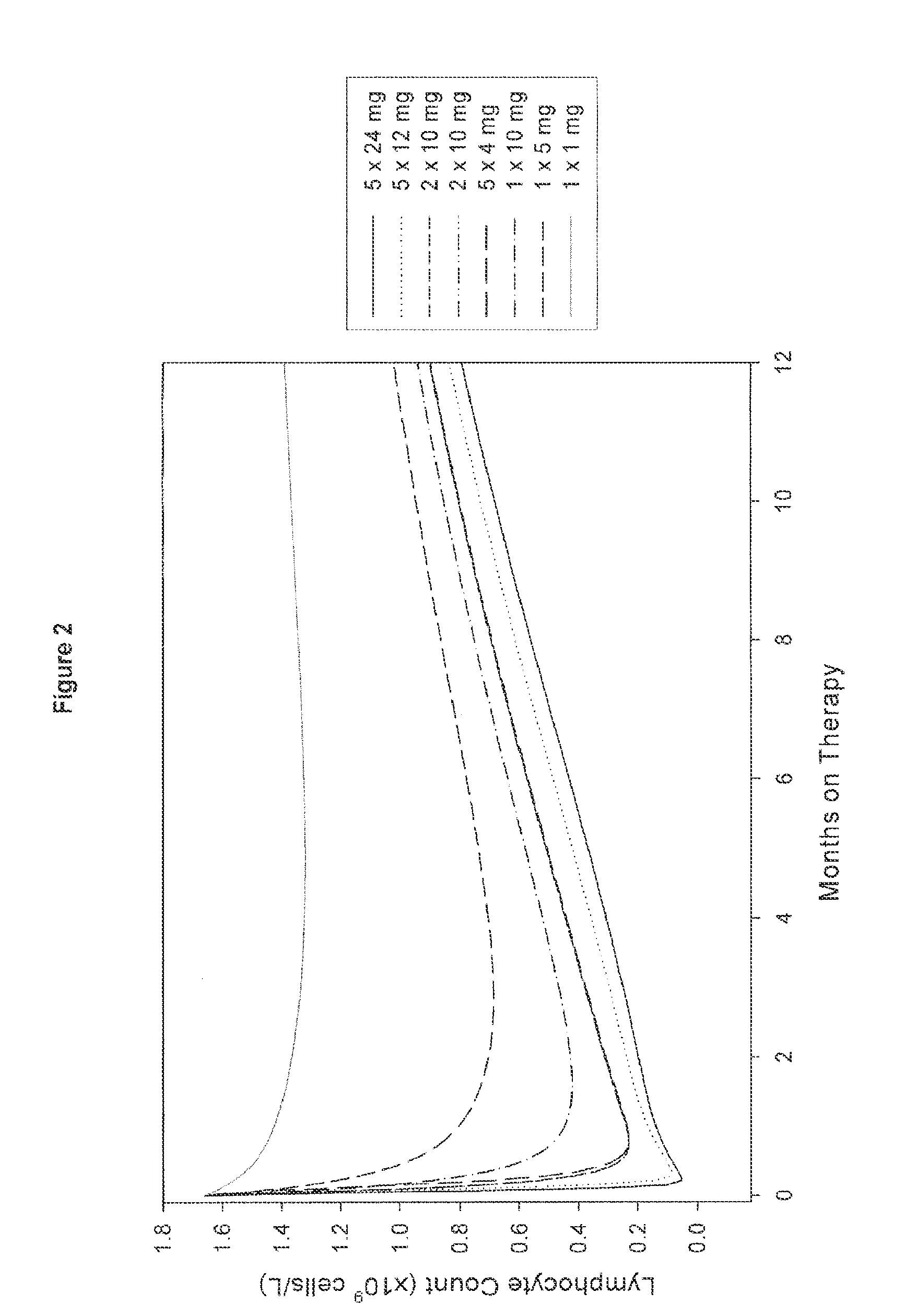
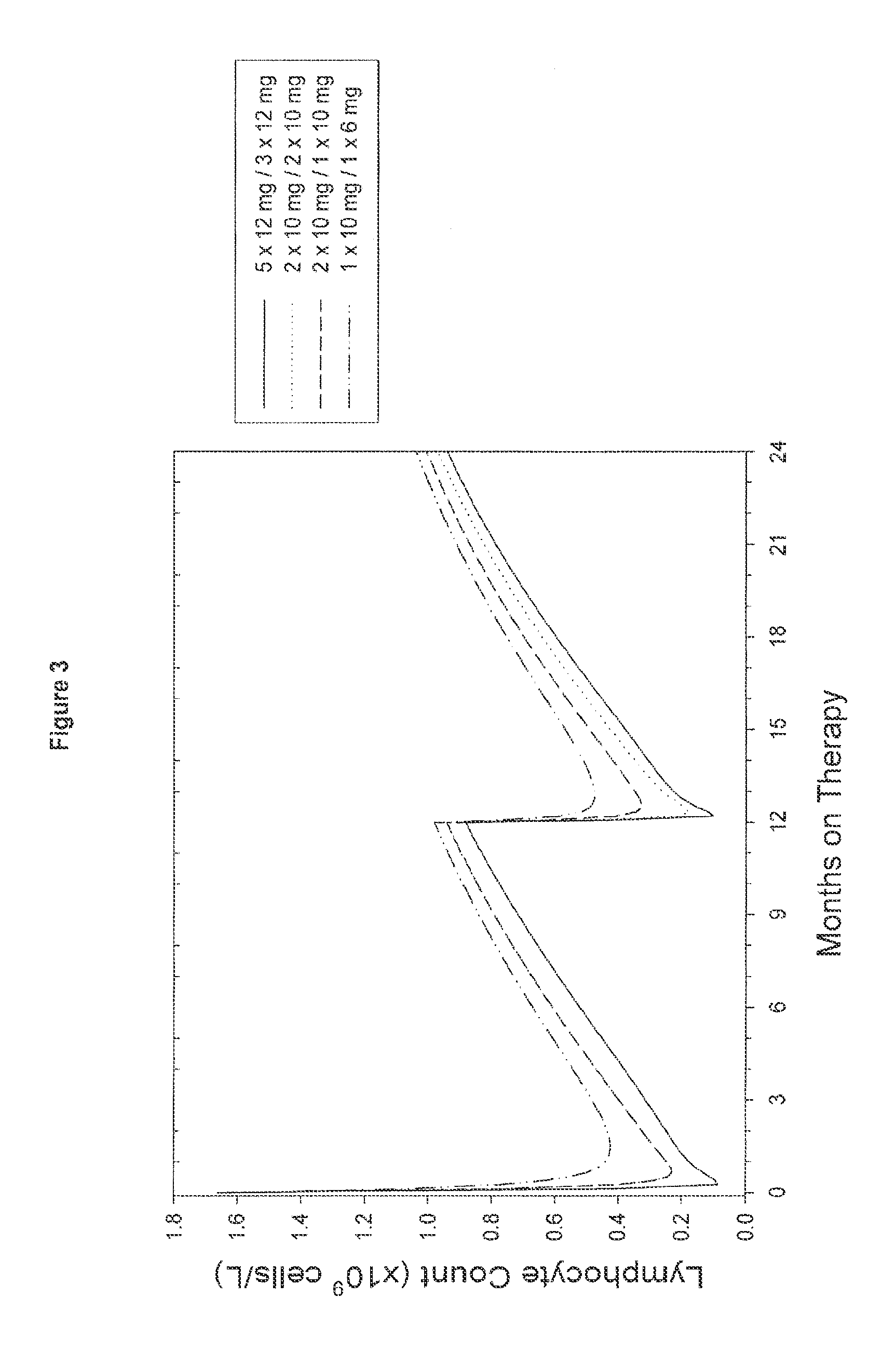
[0080]An open, rater blinded, randomized, multicenter trial in treatment naïve patients with early active relapsing-remitting multiple sclerosis is performed. The first treatment cycle of Campath-1H consists of 5 daily doses of 12 mg at Month 0. The second (fixed) treatment cycle consists of 3 daily doses of 12 mg at Month 12. Subsequently, re-treatment of patients with 3 daily doses of 12 mg Campath-1H only occurs once evidence of renewed MS activity is observed in the respective patient. Thus, the third and subsequent cycles are administered only if the patients have evidence of renewed MS activity, in this example defined as at least 1 documented clinical relapse or presentation of at least 3 new MRI lesions (total) compared to the MRI following the prior Campath-1H treatment (i.e. “re-treatment as needed” regimen). If these criteria are fulfilled, the patient may be immediately retreated.
[0081]If a Campath-1H patient has received steroids for symptomatic treatment of a relapse w...
example 2
[0083]MS patients are treated as set forth above, except that the first treatment cycle of Campath-1H consists of 2 daily doses of 10 mg at Month 0 and the second treatment cycle consists of two daily doses of 10 mg at Month 12. Subsequently re-treatment of patients with 2 daily doses of 10 mg Campath-1H only occurs once evidence of renewed MS activity is observed as described in Example 1 (“re-treatment as needed”).
example 3
[0084]MS patients are treated using the same treatment regimens as outlined in Examples 1 or 2, except that the second and subsequent re-treatments are on a re-treatment as needed basis provided that at least 6 months have passed since the prior Campath-1H treatment cycle (dosing period).
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com