Expression of Hexose Kinase in Recombinant Host Cells
a recombinant host and kinase technology, applied in the field of industrial microbiology and alcohol production, can solve the problems of affecting the redox balance of a recombinant host cell is improved, and the ability of global demand for liquid transportation fuel is projected to strain, so as to improve the redox balance of a recombinant host cell, improve the redox balance of a recombinant hos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Expression Vectors for Isobutanol Pathway Gene Expression in S. cerevisiae
[0244]pLH475-74B8 Construction
[0245]The pLH475-Z4B8 plasmid (SEQ ID NO: 29) was constructed for expression of ALS and KARI in yeast. pLH475-Z4B8 is a pHR81 vector (ATCC #87541) containing the following chimeric genes: A) CUP1 promoter region derived sequence (SEQ ID NO: 30), acetolactate synthase coding region from Bacillus subtilis (AlsS; SEQ ID NOs: 31 and 32) and a CYC1 terminator region derived sequence (“CYC1 terminator 2”; SEQ ID NO: 33); B) ILV5 promoter region derived sequence (SEQ ID NO: 34), Pf5.IlvC-Z4B8 coding region (SEQ ID NOs: 37 and 38) and ILV5 terminator region derived sequence (SEQ ID NO: 35); and C) FBA1 promoter region derived sequence (SEQ ID NO: 36), S. cerevisiae KARI coding region (ILV5; SEQ ID NOs: 39 and 40) and CYC1 terminator region derived sequence.
[0246]The Pf5.IlvC-Z4B8 coding region is a sequence encoding KARI derived from Pseudomonas fluorescens with certain m...
example 2
Pyruvate Decarboxylase and Hexokinase 2 Gene Inactivation
[0255]This example describes insertion-inactivation of endogenous PDC1, PDC5, and PDC6 genes of S. cerevisiae. PDC1, PDC5, and PDC6 genes encode the three major isozymes of pyruvate decarboxylase. The resulting PDC inactivation strain was used as a host for expression vectors pLH475-Z4B8 and pLH468 that were described in Example 1.
Construction of pdc6::PGPM1-sadB Integration Cassette and PDC6 Deletion:
[0256]A pdc6::PGPM1-sadB-ADH1t-URA3r integration cassette was made by joining the GPM-sadB-ADHt segment (SEQ ID NO: 64) from pRS425::GPM-sadB (described above) to the URA3r gene from pUC19-URA3r. pUC19-URA3r (SEQ ID NO: 65) contains the URA3 marker from pRS426 (ATCC #77107) flanked by 75 bp homologous repeat sequences to allow homologous recombination in vivo and removal of the URA3 marker. The two DNA segments were joined by SOE PCR (as described by Horton et al. (1989) Gene 77:61-68) using as template pRS425::GPM-sadB and pUC19...
example 3
Production of Isobutanol
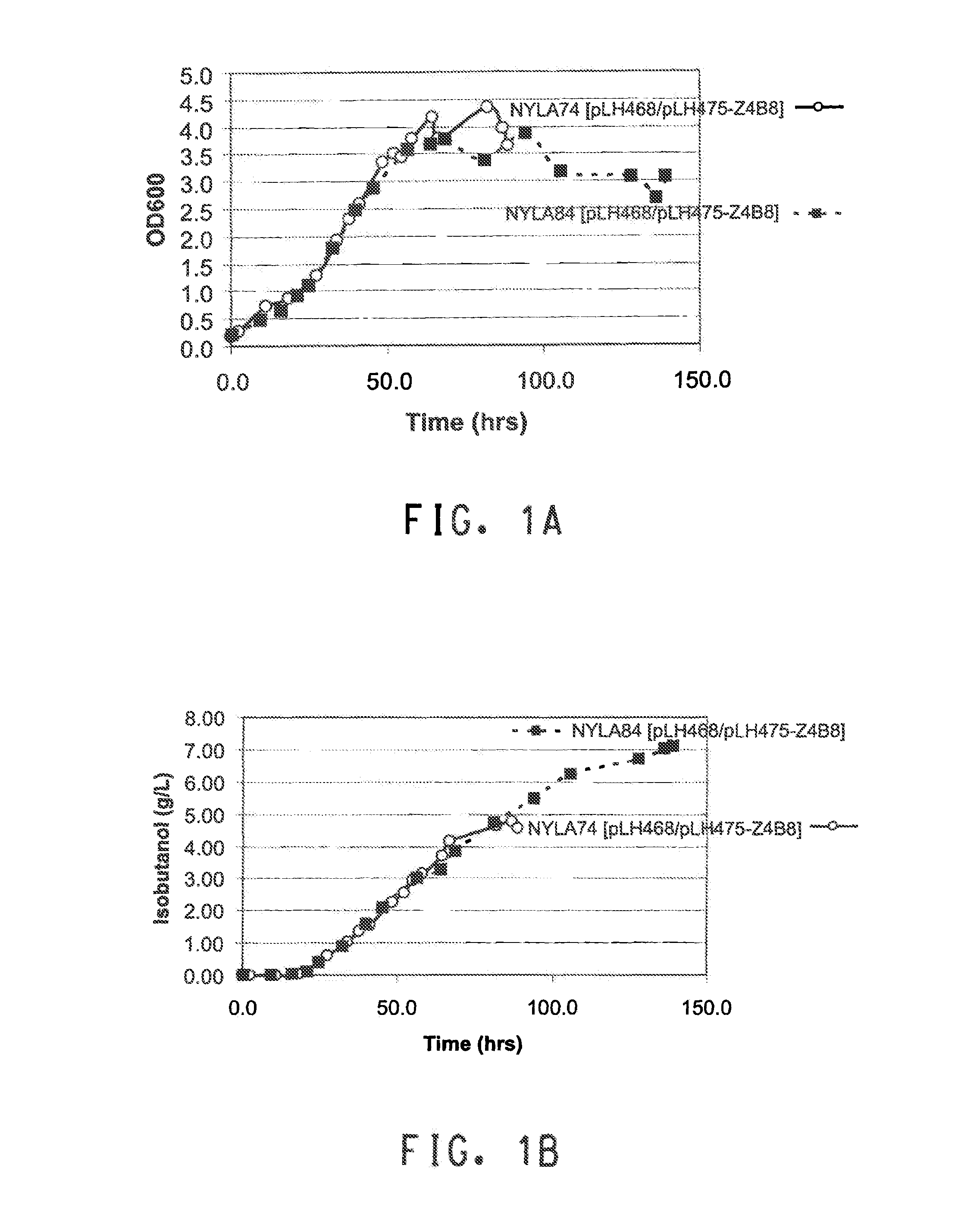
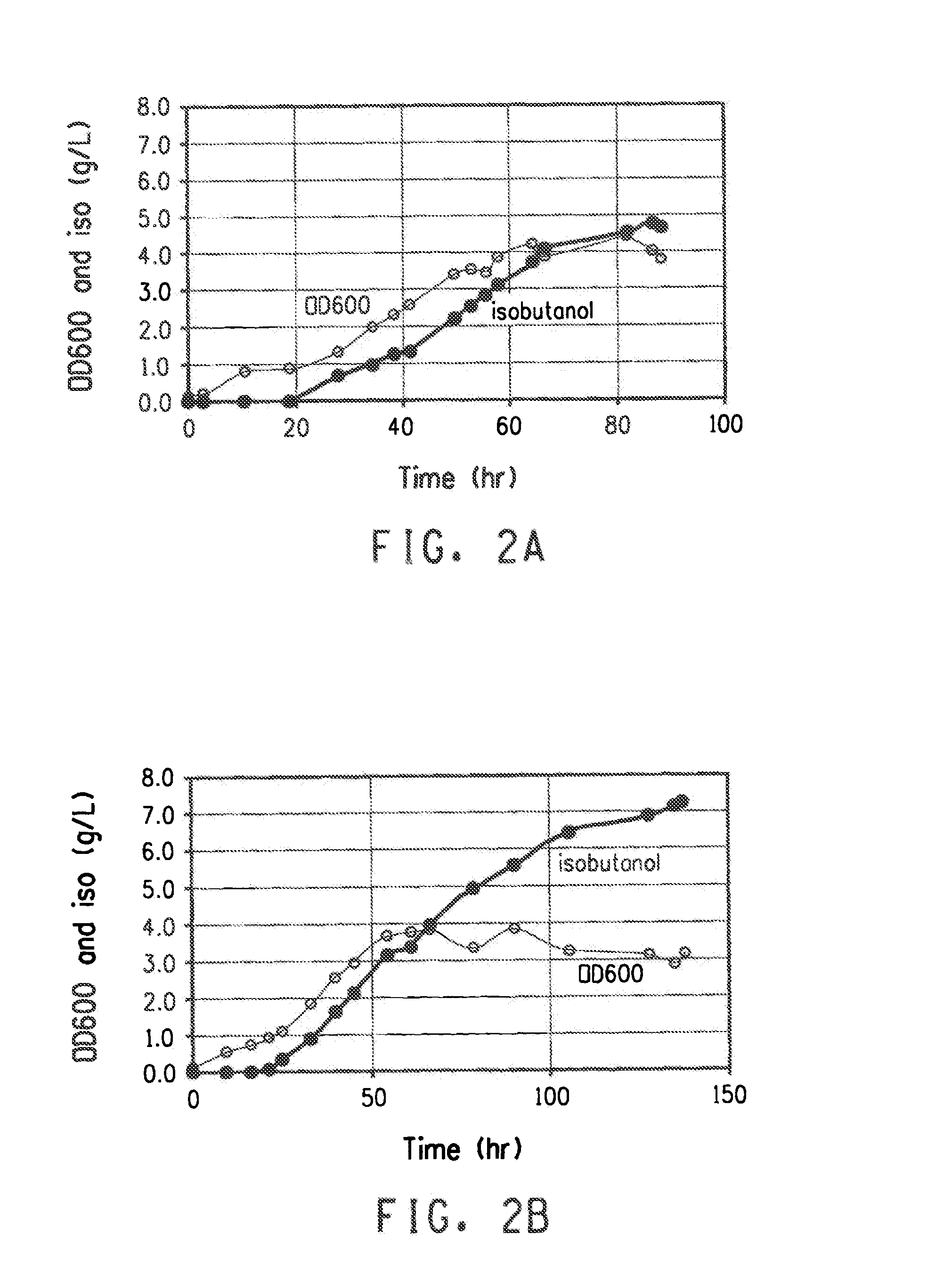
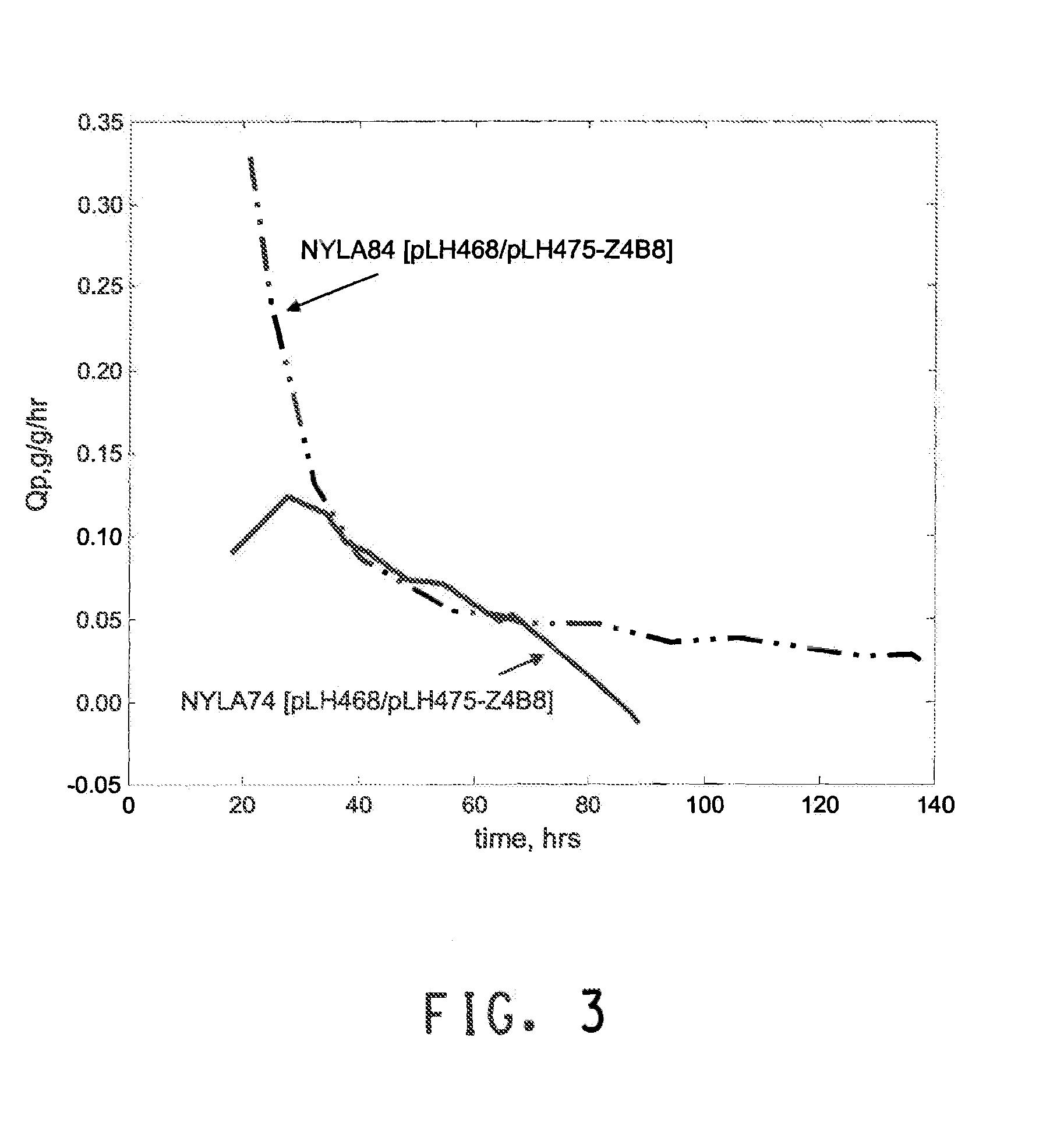
[0264]The purpose of this example is to describe the production of isobutanol in the yeast strain NYLA84. The yeast strain comprises deletions of PDC1, PDC5, and PDC6, genes encoding three isozymes of pyruvate decarboxylase, and constructs for heterologous expression of AlsS (acetolactate synthase), KARI (keto acid reductoisomerase), DHAD (dihydroxy acid dehydratase), KivD (ketoisovalerate decarboxylase), and SadB (secondary alcohol dehydrogenase).
Strain Construction
[0265]Plasmids pLH468 and pLH475-Z4B8 were introduced into NYLA74 or NYLA84, described in Example 2, by standard PEG / lithium acetate-mediated transformation methods. Transformants were selected on synthetic complete medium lacking glucose, histidine and uracil. Ethanol (1% v / v) was used as the carbon source. After three days, transformants were patched to synthetic complete medium lacking histidine and uracil supplemented with both 2% glucose and 1% ethanol as carbon sources. Fermentation seed via...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com