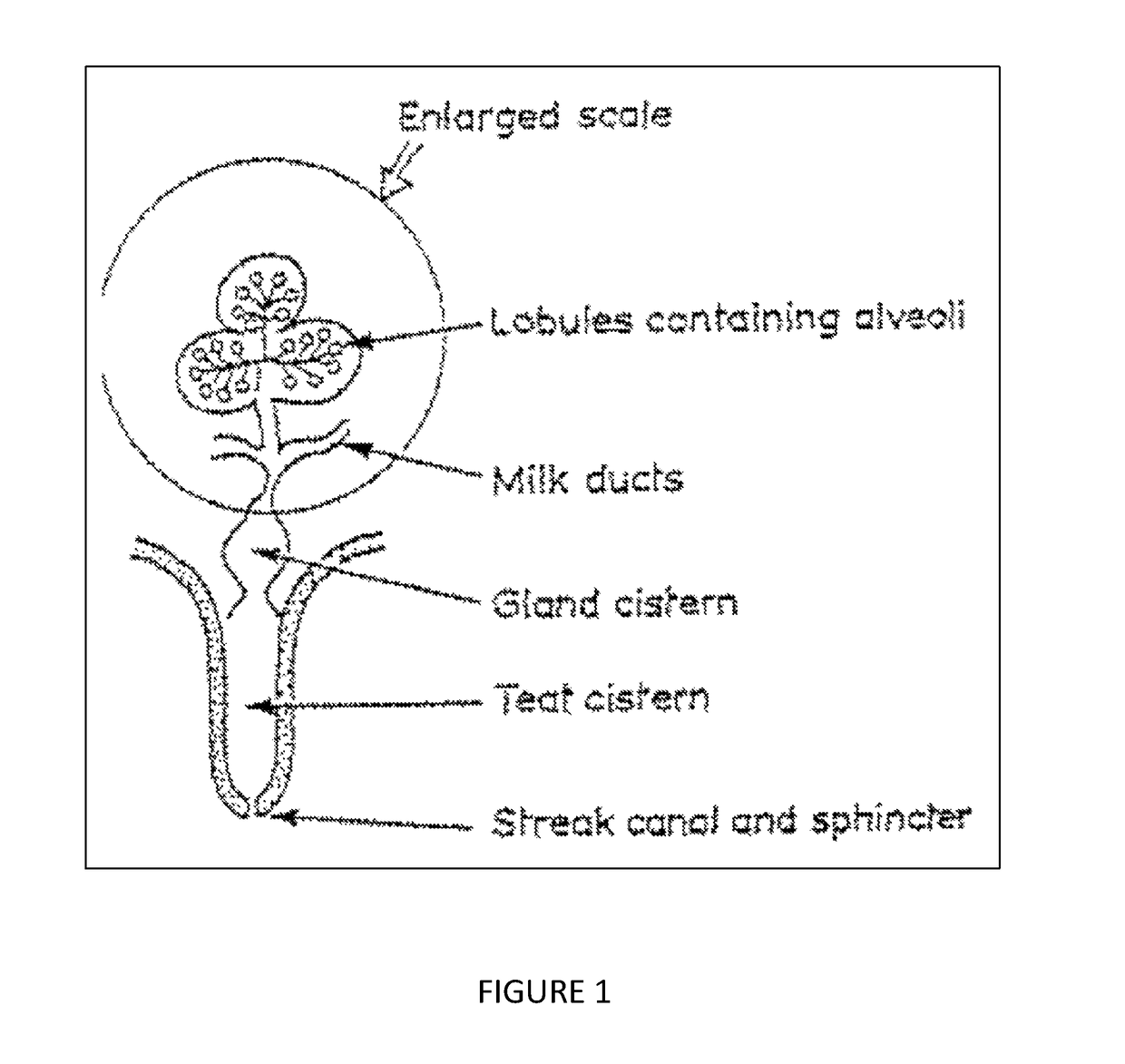
Hydrogel formulation with mild adhesion
a technology of hydrogel and mild adhesion, which is applied in the direction of antibacterial agents, capsules, aerosol delivery, etc., can solve the problems of hydrogel shrinkage, significant shrinkage, and hydrogel properties change, and achieve the effect of reducing the period of tim
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulations
[0277]Hydrogel formulations were prepared from two solutions, an oxidant solution and a reductant solution, which were combined to form the hydrogel. The two solutions contained some ingredients in equal parts (macromer and glycerol) but only the oxidant solution contained hydrogen peroxide and only the reductant solution contained Fe(II), ascorbic acid, and calcium chloride.
[0278]The components of the oxidant were mixed into a homogeneous solution. Once fully mixed, the oxidant component was dispensed into one part of a dual barrel syringe. The components of the reductant were similarly mixed into a homogenous solution. After thorough mixing, the reductant component was dispensed into the second part of the dual barrel syringe.
[0279]The macromers used in the formulations were PVA of the molecular weights noted, substituted with the noted amounts of N-acrylamidoacetaldehyde dimethyl acetal (NAAADA). The macromers were prepared applying the method of Example 15 from U.S. ...
example 2
Swelling and Adhesion Testing
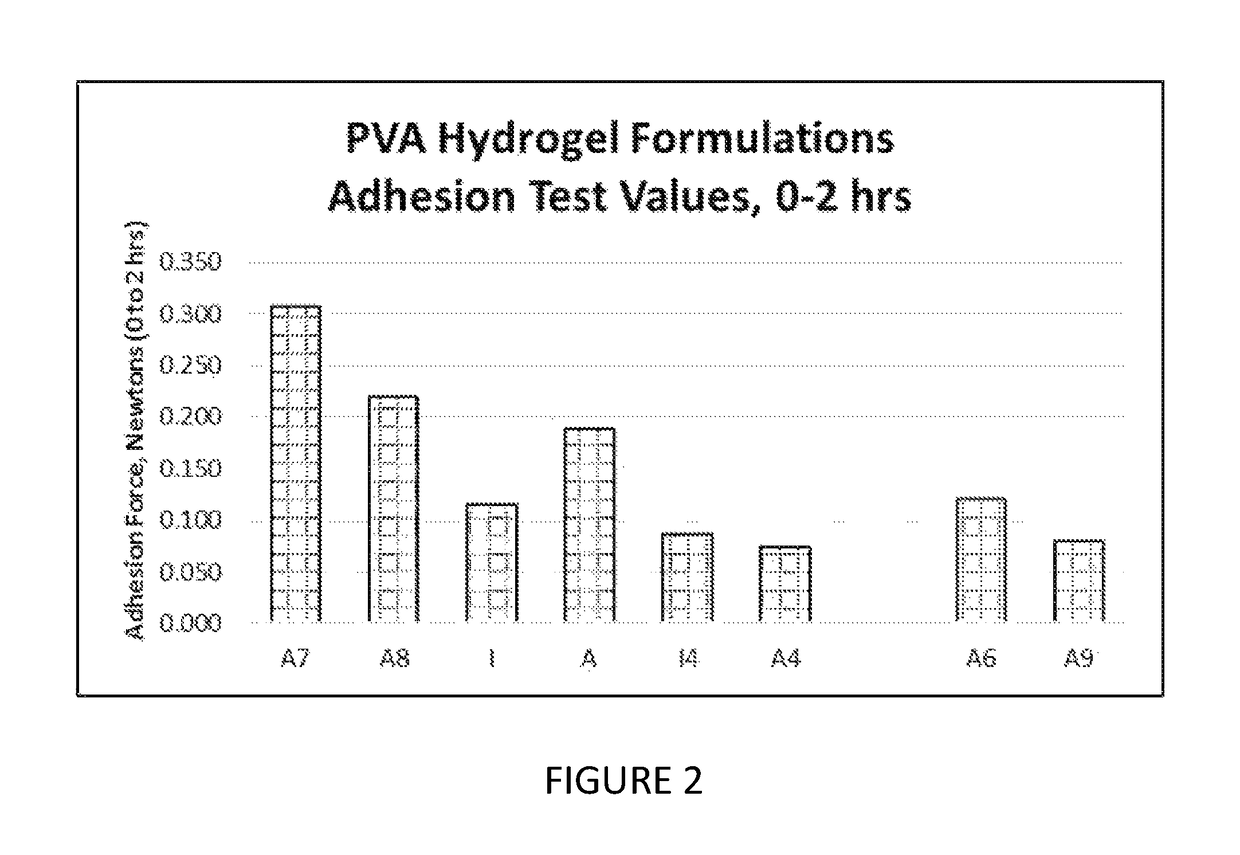
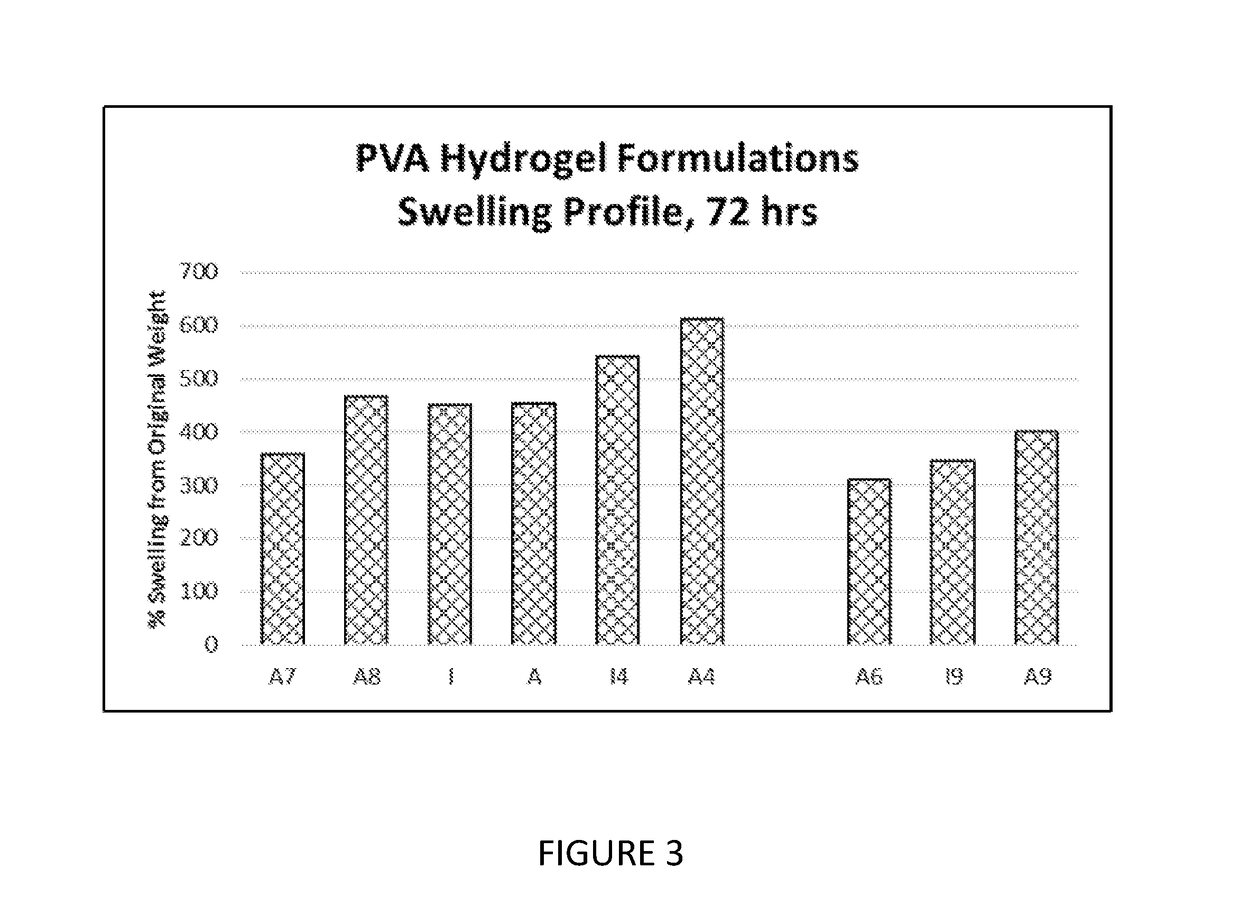
[0284]For adhesion testing, samples were conditioned at 37° C. for 2 hrs prior to testing. Adhesion data were generated between 0 to 2 hours after in situ formation of the hydrogel composition. The swelling values provided herein were read after 72 hours of swelling time. Swelling to the recorded values occurred during the noted time frame, and it is understood that readings taken between in situ formation and the final reading time would have been less than the value recorded at 72 hours.
[0285]For adhesion testing, peel test samples were made using a 60 mm×50 mm×3 mm mold attached to damp (or wet) collagen with an affixed gauze backing to facilitate the peeling. To assess adhesion on these samples, a modified procedure from the standard 180° peel test was performed with the principal modification of setting the sample horizontally as opposed to vertically. A high-precision universal peel tester was used to measure the force to pull the backing “on itsel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adhesion peel test pull force | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| adhesion peel test pull force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com