Anti-proliferative combination therapy comprising satraplatin or JM118 and a taxane
A taxane and docetaxel technology, which can be used in anti-tumor drugs, drug combinations, active ingredients of heavy metal compounds, etc., can solve problems such as no implied therapeutic application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0289] The term "preparation of [the first] pharmaceutical composition" refers to any step or method performed or required in the manufacture of a pharmaceutical composition that is conveniently administered to a patient or individual in need thereof. The steps or methods include the preparation of the pharmaceutical composition, formulation of the pharmaceutical composition into a formulation, packaging of the pharmaceutical composition and other steps performed before the pharmaceutical composition is delivered, sought or provided to a pharmacist, doctor or nurse. It also includes methods and procedures performed by a pharmacist, physician or nurse prior to administration of the pharmaceutical composition. For example, the above-mentioned methods and steps performed by a pharmacist, doctor or nurse include dissolving the pharmaceutical composition in a suitable solvent for administration, such as injection; Administering or other steps permitting administration of the pharma...
example 1
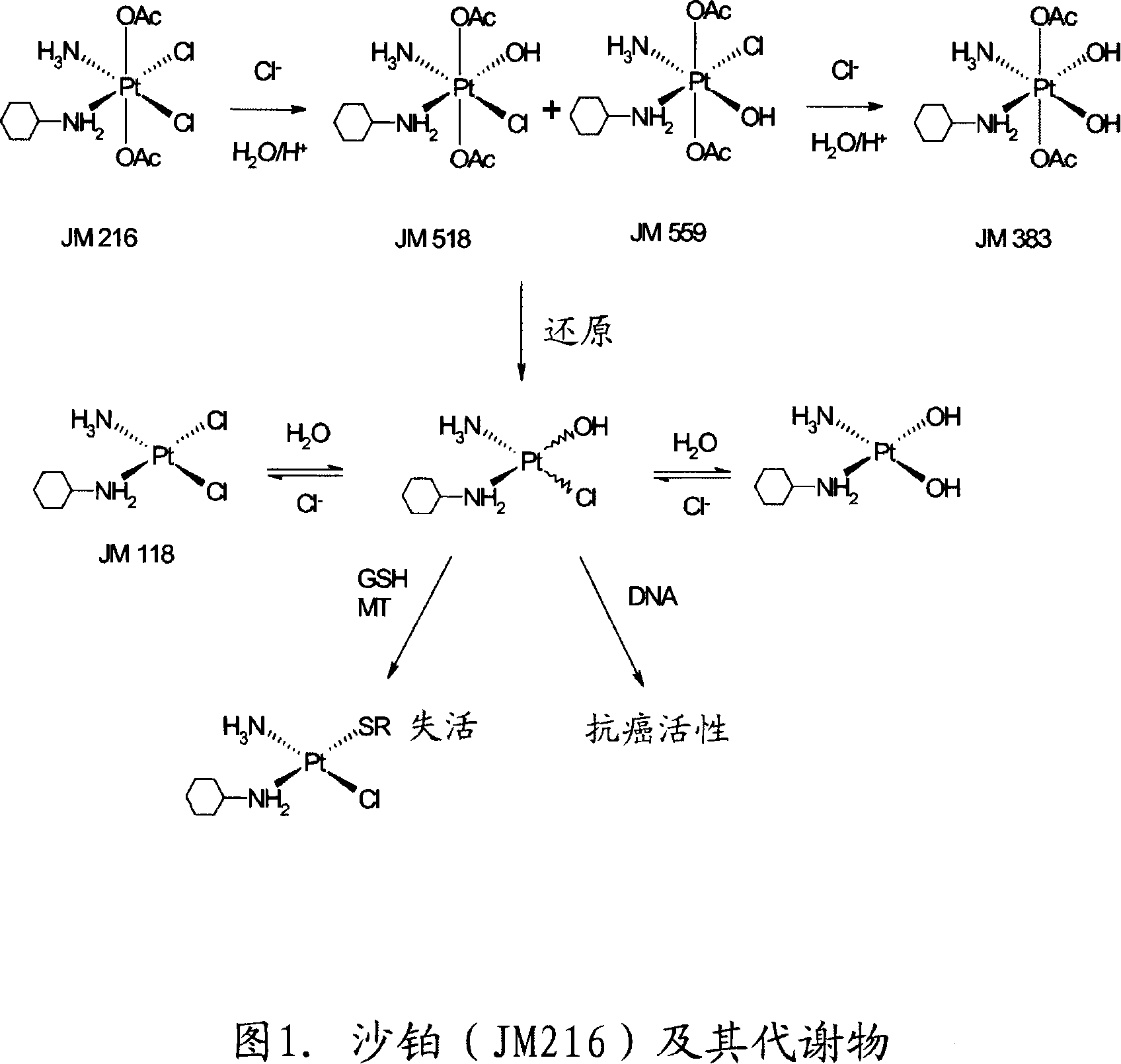
[0328] Example 1. The effectiveness of satraplatin and its metabolites in cisplatin-resistant tumor cells
[0329] The inventors have observed the surprising result that the platinum-based compounds of the invention can be used to inhibit or kill tumor cells resistant to other platinum compounds such as cisplatin.
[0330] The A129 cp80 cell line (obtained by Tito Foio, NIH; BiochemPharmacol52, 1855) produced by ovarian tumor A2780 is highly resistant to cisplatin (in individual experiments, the relative resistance ranges between 80-106 times), but to Treatment with JM216, JM118 and JM383 remained susceptible (relative tolerability ranged between 0.19-2.59 fold in individual experiments (Table 1)). The parent non-variant cell line A129 was used as a control.
[0331] 1,000-5,000 cells per well were exposed to various concentrations of test compounds for 48 hours in order to calculate the IC50 values shown in Table 1. Cytotoxicity was measured using the SRB method accordi...
example 2
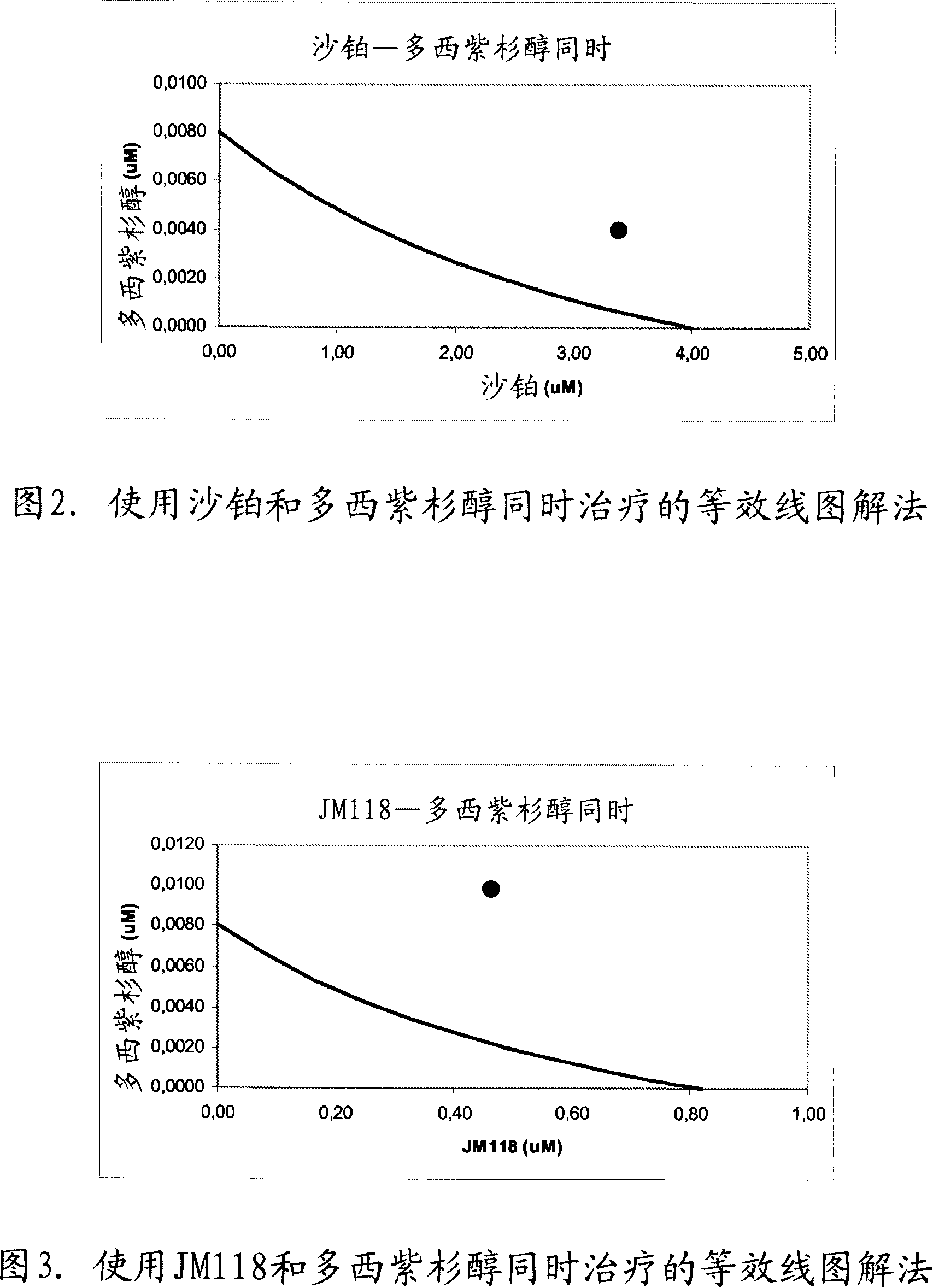
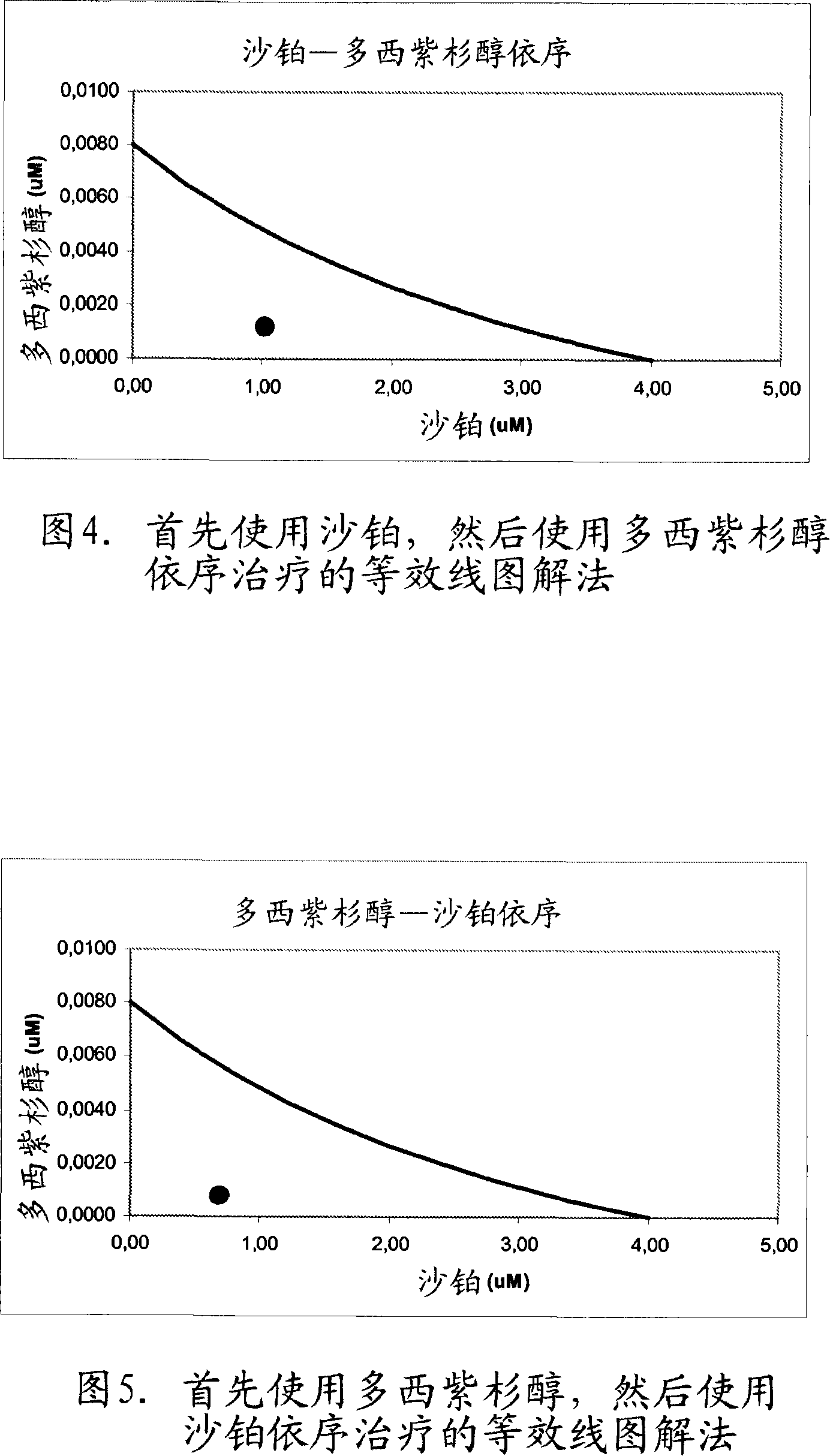
[0335] Example 2. Synergy between platinum-based compounds and docetaxel
[0336] The inventors have observed the surprising result that a combination of platinum-based compounds, particularly satraplatin and JM118 and docetaxel, acts synergistically when sequentially exposed or contacted to cancer cells or tumor cells.
[0337] The prostate adenocarcinoma cell line PC-3 (ATCC accession number: CRL-1435; Invest Urol (1979) 17, 16; Cytogenet Cell Genet (1993) 62, 183) was used. PC-3 cells were obtained from non-confluent plates and seeded in 96-well plates at a density of 2,000 cells per well. At 37°C, 5% CO 2 The cells were cultured in F-12K medium supplemented with 10% FCS and 1% Pen / Strep under the condition of . Twenty-four hours after addition to the plate, cells were exposed to a range of concentrations of individual compounds and incubated for 48 hours. Cytotoxicity was then measured using the SRB method according to Shekan et al. (Example 1; J Natl Cancer Inst (199...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com