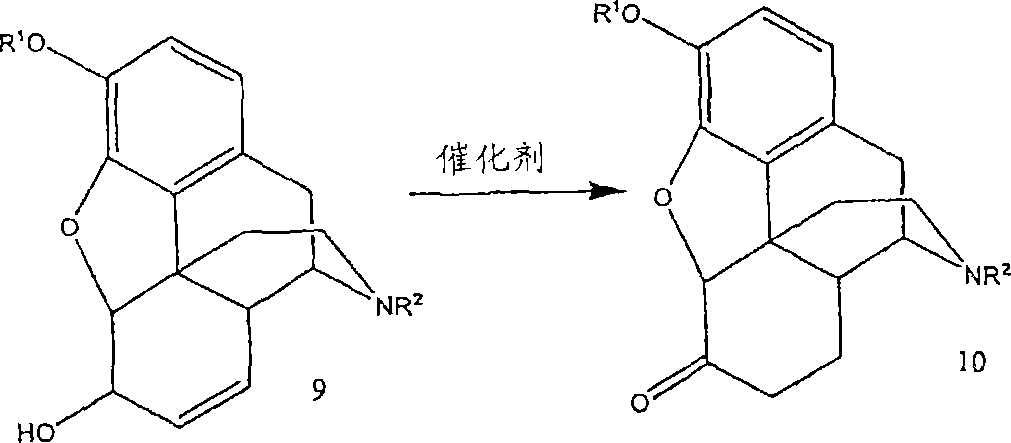
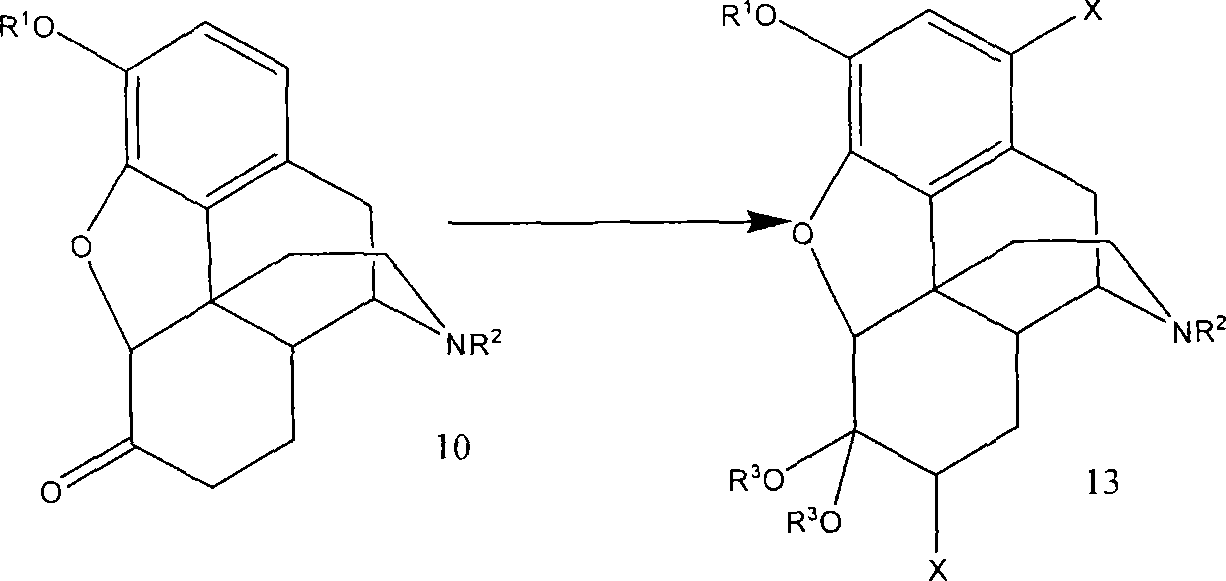
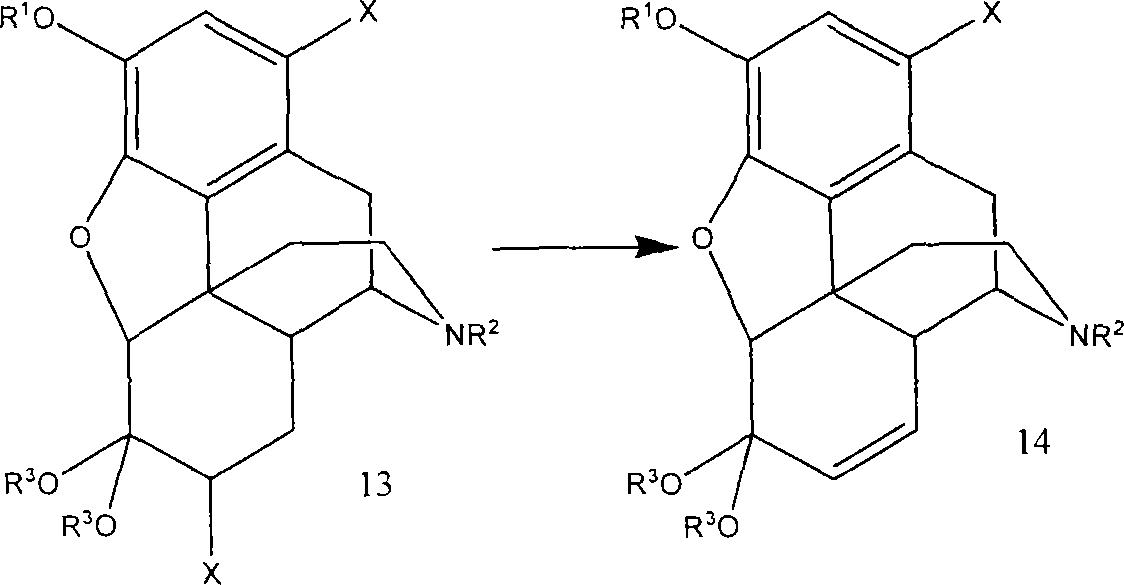
A new synthetic route to 14-hydroxyl opiates through 1-halo-thebaine or analogs
一种芳基、苄基的技术,应用在制备14-羟基-阿片类,[0001]本发明涉及制备14领域,能够解决低收率、不易分离、动力学差等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] To a 1000 mL three-necked flask was added MeOH (250 mL). Add codeine (62.5 g, 0.209 mol) and stir until completely dissolved. The solution was bubbled with nitrogen for 10 minutes. Add Wilkenson catalyst Rh (PPh 3 ) 3 Cl (0.625g). The solution was refluxed for 3 h to obtain a suspension. MeOH (125 mL) was distilled off. The suspension was cooled to 50°C. Join CH(OMe) 3 (125 mL) and H 2 SO 4 (14.2 mL). After joining H 2 SO 4 Keep the temperature below 62°C during the process. After reflux for 30 minutes, the solution was cooled to 50°C. Add Br within 45 minutes 2 (23.63 mL, 0.46 mol) of CHCl 3 (125 mL) solution. The temperature was maintained at or near 50° C. for 30 minutes after the addition was complete. The solution was filtered. The filtrate and CHCl 3 (125 mL) was added to the stirred 29% NH 4 OH (188 mL) and water (188 mL) at 0-5°C for about 10 minutes until two layers formed. CHCl for aqueous layer 3 (125 mL) for extraction. The organic lay...
Embodiment 2
[0070] Dissolve 1,7-dibromohydrocodone dimethyl ketal (13, 80.0g, 0.159mmol) in 1-methyl-2-pyrrolidone (NMP, 120mL), blow nitrogen gas for 10 minutes, and cool to 5 ~10°C. KOBu-t (26.8 g, 0.24 mol) was added in three portions. Due to the exotherm of the reaction, the temperature was maintained below 45°C by controlling the rate of addition. After the addition the mixture was stirred at 40-45°C for 1 hour and then cooled to room temperature. Glacial acetic acid (HOAc, 180 mL) was then added. The solution was heated to 100°C for 6 hours. HOAc (-100 mL) was removed by distillation. The solution was poured into ice water (480 mL), followed by washing with toluene (120 mL). Toluene (480 mL) was added to the aqueous layer. NaOH (50%) was added until pH = 12.3. The organic layer was washed with water (5 x 480 mL). The product in the organic layer was found to contain 41.5 g (purity 88% area / area by HPLC). The crude product 21 was isolated as a tan solid (45 g).
Embodiment 3
[0072] Compound 15 (5.00 g, 12.8 mmol) was dissolved in HOAc (15 mL). Add peracetic acid (CH) dropwise at 25-30°C 3 CO 3 H~20%, by HOAc / H 2 O freshly prepared, 7.2mL, ~19mmol). Stirring was continued at room temperature for 50 minutes after the addition was complete. 5% Pd / C (0.25 g) was added, stirred at room temperature for 2 hours, and then 5% Pd / C (0.75 g) was added again. The reactor was flushed 3 times with nitrogen and then 3 times with hydrogen. The reactor was heated to 60°C under 60 psi hydrogen for 12 hours. The suspension is filtered. The solid was washed with MeOH (2 x 5 mL). The combined filtrates were evaporated to essentially dryness under reduced pressure. Add ethyl acetate (20 mL) / 5% NH 4 OH (20 mL, final pH > 10). The aqueous layer was extracted with ethyl acetate (20 mL). The combined organic layers were washed with water (3 x 10 mL) and evaporated to dryness under reduced pressure to give 3.3 g of crude product 17 as a solid. Compound 17 was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com