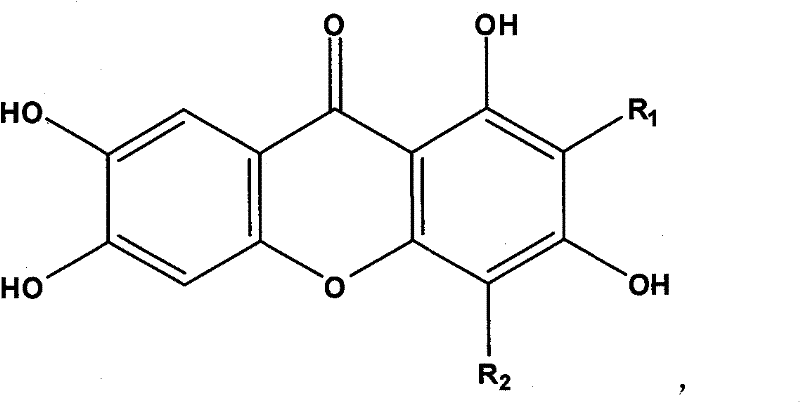
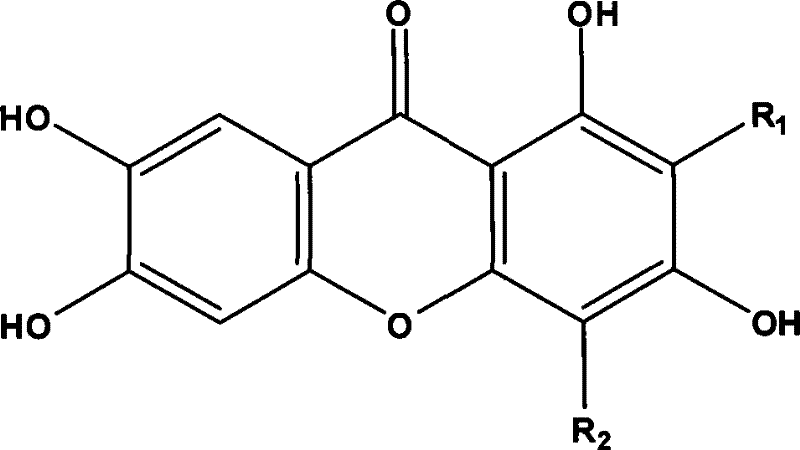
New use of mangiferin compounds
A technology of mangiferin and compounds, applied in the field of medicine, can solve problems such as gout and hyperuricemia that have not been seen before
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Effects of mangiferin, isomangiferin and norathyriol on hyperuricemia mice
[0013] Healthy male Kunming mice, weighing 18-22 g, were provided by the Experimental Animal Center of Kunming Medical College [Experimental Animal Production License Number: SCXK (Dian) 2005-2008]. Breeding conditions: room temperature is 22±2°C, relative humidity is 60-70%.
[0014] Fifty Kunming mice were randomly divided into normal control group, hyperuricemia model group, mangiferin, isomangiferin and norathyriol groups. The test compound was prepared into a suspension with 0.5% sodium carboxymethylcellulose (0.5% CMC-Na), and administered by intragastric administration at 3.13 mg / kg, twice a day, 5 doses in total.
[0015] The uricase inhibitor oxonic acid is selected as a chemical inducer to inhibit the activity of uricase and cause hyperuricemia. Animals were intraperitoneally injected mice with oxonic acid potassium salt (product of Sigma Company) at a dose of 400 mg / kg 2 h before b...
Embodiment 2
[0021] Study on the dose-effect relationship of mangiferin in reducing hyperuricemia
[0022] Seventy healthy male Kunming mice, weighing 18-22 g, were provided by the Experimental Animal Center of Kunming Medical College [Experimental Animal Production License Number: SCXK (Dian) 2005-2008]. Animals were randomly divided into normal control group, hyperuricemia model group, mangiferin 0.88, 1.56, 3.13 and 6.25 mg / kg dose groups and allopurinol (3.13 mg / kg) positive control group. The test compound was prepared into a suspension with 0.5% sodium carboxymethylcellulose (0.5% CMC-Na), administered orally, twice a day, 5 doses in total.
[0023] The hyperuricemia modeling method is the same as before, that is, the mice are intraperitoneally injected with 400 mg / kg oxonic acid potassium salt 2 hours before blood sampling to cause hyperuricemia, and the normal control group is injected with an equal volume of 0.5% CMC-Na solution, and irrigated after 1 hour. The last dose of the t...
Embodiment 3
[0028] The influence of embodiment 3 mangiferin on xanthine oxidase
[0029] Xanthine oxidase (EC 1.1.3.22) is a product of Sigma Company, prepared with PBS to an appropriate concentration for use. Mangiferin was prepared into a series of concentrations with PBS for use, and DMSO was used to aid in dissolution. Xanthine oxidase assay kit is a product of Nanjing Jiancheng Bioengineering Institute.
[0030] After incubating the mangiferin solution with serial concentrations and xanthine oxidase at 37°C for 30 minutes, the activity of xanthine oxidase was measured with a xanthine oxidase assay kit. The results showed that mangiferin had a certain inhibitory effect on the activity of xanthine oxidase ( table 3).
[0031] Table 3: Effect of mangiferin on xanthine oxidase (x±s, n=4)
[0032] group
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com