Use of a topical composition containing epidermal growth factor (EGF) for diabetic foot amputation prevention
An epidermal growth factor, topical technology, used in medical preparations containing active ingredients, drug combinations, skin diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1. Production of EGF-loaded liposomes
[0022] Phosphatidylcholine was dissolved at a concentration of 10 mg / mL in absolute ethanol in a 50 mL round bottom flask. Solvent was removed by rotary evaporation until a dry lipid film formed on the flask walls. For encapsulation of EGF in liposomes, the dried lipid film was hydrated with a buffered aqueous solution containing EGF and homogenized by stirring. To reduce the size of the vesicles, the suspension was subjected to several extrusions through a 100 nm pore size polycarbonate membrane until the average size of the vesicles was about 100 nm. The EGF-loaded liposome suspension was centrifuged at 100 000 x g for 40 min at 4°C to separate non-encapsulated EGF from encapsulated EGF. The supernatant was transferred to a new tube and the pellet was resuspended in pH 7.2 phosphate buffered saline. The centrifugation step was repeated again under the same conditions and the supernatant was transferred to a new tube a...
Embodiment 2
[0025] Embodiment 2. Determination of the encapsulation efficiency of EGF in liposome
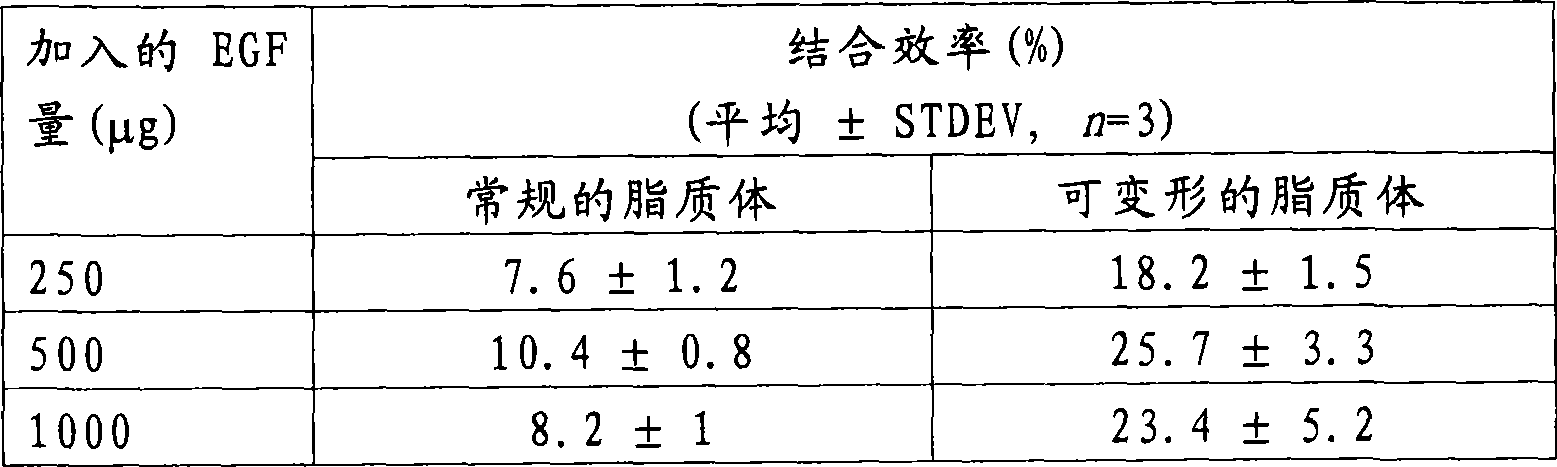
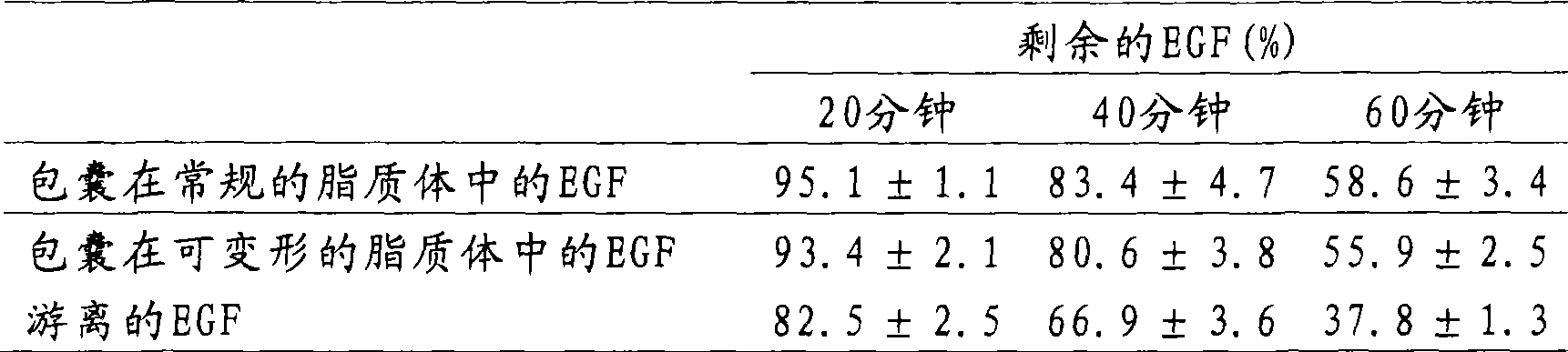
[0026] To determine the incorporation efficiency of EGF in liposomes, liposome suspensions were high-speed centrifuged and the protein content of the resulting pellet (EGF-loaded liposomes) and supernatant (free EGF) was determined. The liposome suspension was centrifuged at 100 000 x g for 40 min at 4 °C. The supernatant was transferred to a new tube and the pellet was resuspended in pH 7.2 phosphate buffered saline. The centrifugation step was repeated again under the same conditions and the supernatant was transferred to a new tube and mixed with the supernatant of the first centrifugation step. The pellet (comprising EGF-loaded liposomes) was resuspended in 500 μL of pH 7.2 phosphate-buffered saline. Then, the protein of the pellet or supernatant was extracted and separated from the lipid by adding 0.5% (v / v) Triton X-100 to the sample, and they were subjected to reverse phase high pe...
Embodiment 3
[0029] Example 3. Determination of liposome size and morphology
[0030] Liposome samples were analyzed by transmission electron microscopy to determine liposome size and morphology. Liposomes were visualized by negative staining with uranyl acetate. Negatively stained samples were observed under a transmission electron microscope Jeol-JEM 2000EX operated at 80KV. Electron micrographs corresponding to each liposome preparation were digitized using a scanner and the diameter of the liposomes was measured using DIGIPATVersion 3.3 software (EICSOFT, Havana, Cuba). Particle size is the average value of all liposomes present in the respective micrographs and is expressed as the mean ± standard deviation of three individual determinations.
[0031] Preparations of deformable or conventional liposomes loaded with EGF consisted of homogeneous populations of spherical or oval vesicles. The average size of the vesicles was 130±7 nm and 123±4 nm for regular and deformable liposomes, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com