Preventive/remedy for cancer
A technology of preventive and therapeutic agents, applied in the field of cancer prevention or therapeutic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
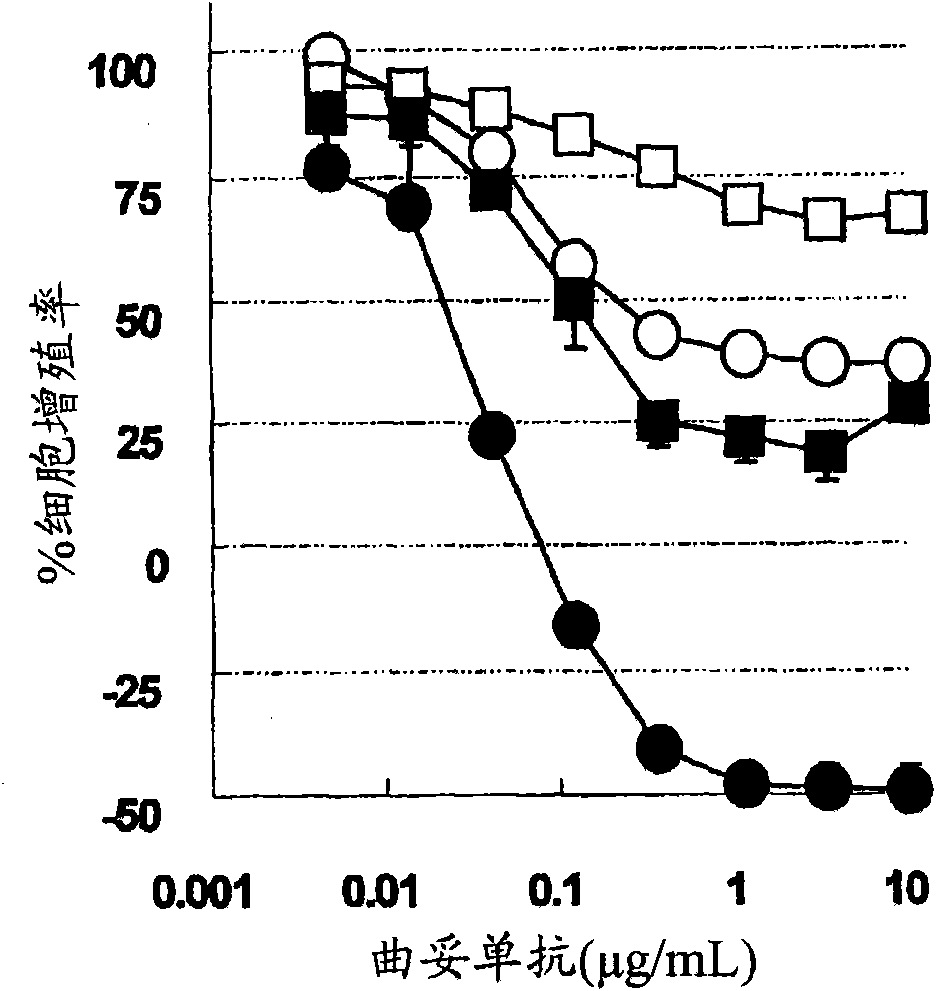
[1291]BT474 cells with high sensitivity to trastuzumab (American Type Culture Collection, HTB-20, J Natl Cancer Inst 61:967-978 (1978)), and BT-474 cells with low sensitivity to trastuzumab were used , the BT-474 cells with low sensitivity to trastuzumab were cultured for 3 months by culturing the BT474 cells with high sensitivity to trastuzumab in RPMI complete medium containing 5 μg / mL trastuzumab or longer. The LIMK1 gene was knocked down by the following RNAi method. Three kinds of Stealth RNAi corresponding to LIMK1 gene (LIMK1 Stealth Select 3 RNAi, Invitrogen Company, HSS140837: HSS140836: HSS141043) and LipofectaminRNAiMAX (Invtrogen Company, 13778-150) were in Opti-MEM(R)I reduced serum medium ( reduced-serum medium) (Invitrogen, 31985-070) to 36.7nMx3 (110nM in total) and 11μL / mL, respectively. After standing at room temperature for 20 minutes, the Stealth RNAi was mixed with each BT-474 cell suspension adjusted to 22,000 cells / mL in the culture medium at a liquid ...
Embodiment 2
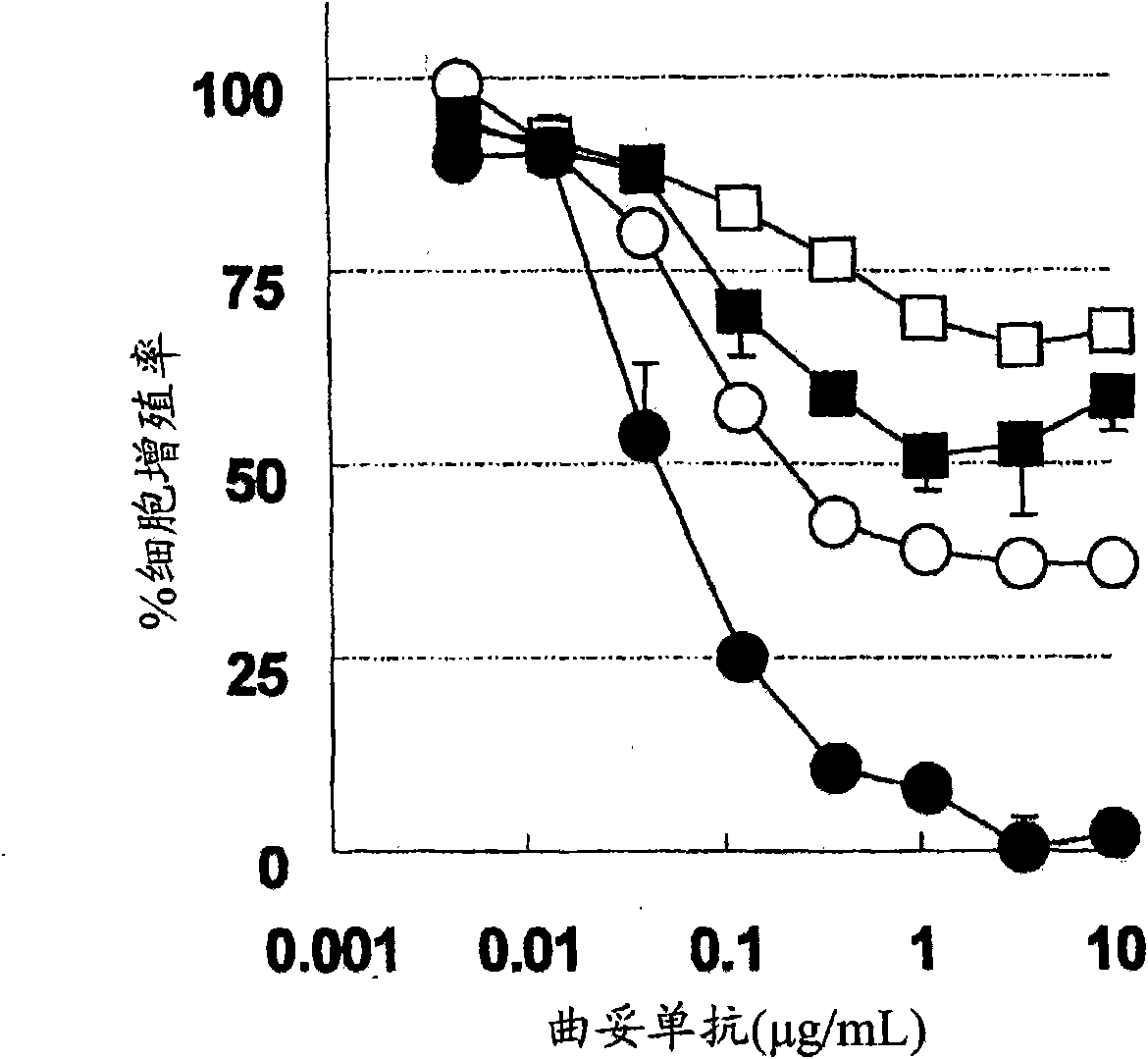
[1294] BT474 cells with high sensitivity to trastuzumab (American Type Culture Collection, HTB-20, J Natl Cancer Inst 61:967-978 (1978)), and BT-474 cells with low sensitivity to trastuzumab were used , the BT-474 cells with low sensitivity to trastuzumab were cultured for 3 months by culturing the BT474 cells with high sensitivity to trastuzumab in RPMI complete medium containing 5 μg / mL trastuzumab or longer. The PAK1 gene was knocked down by the following RNAi method. Two kinds of Stealth RNAi (PAK1 Validated Stealth RNAi DuoPac, Invitrogen Company, 45-1676) and Lipofectamin RNAiMAX (Invtrogen Company, 13778-150) corresponding to the PAK 1 gene were in Opti-MEM(R)I reduced serum medium ( Invitrogen Company, 31985-070) were mixed to 55nM x2 (a total of 110nM) and 11μL / mL. After standing at room temperature for 20 minutes, the Stealth RNAi was mixed with each BT-474 cell suspension adjusted to 22,000 cells / mL in the culture medium at a liquid volume ratio of 1:11 (final con...
Embodiment 3
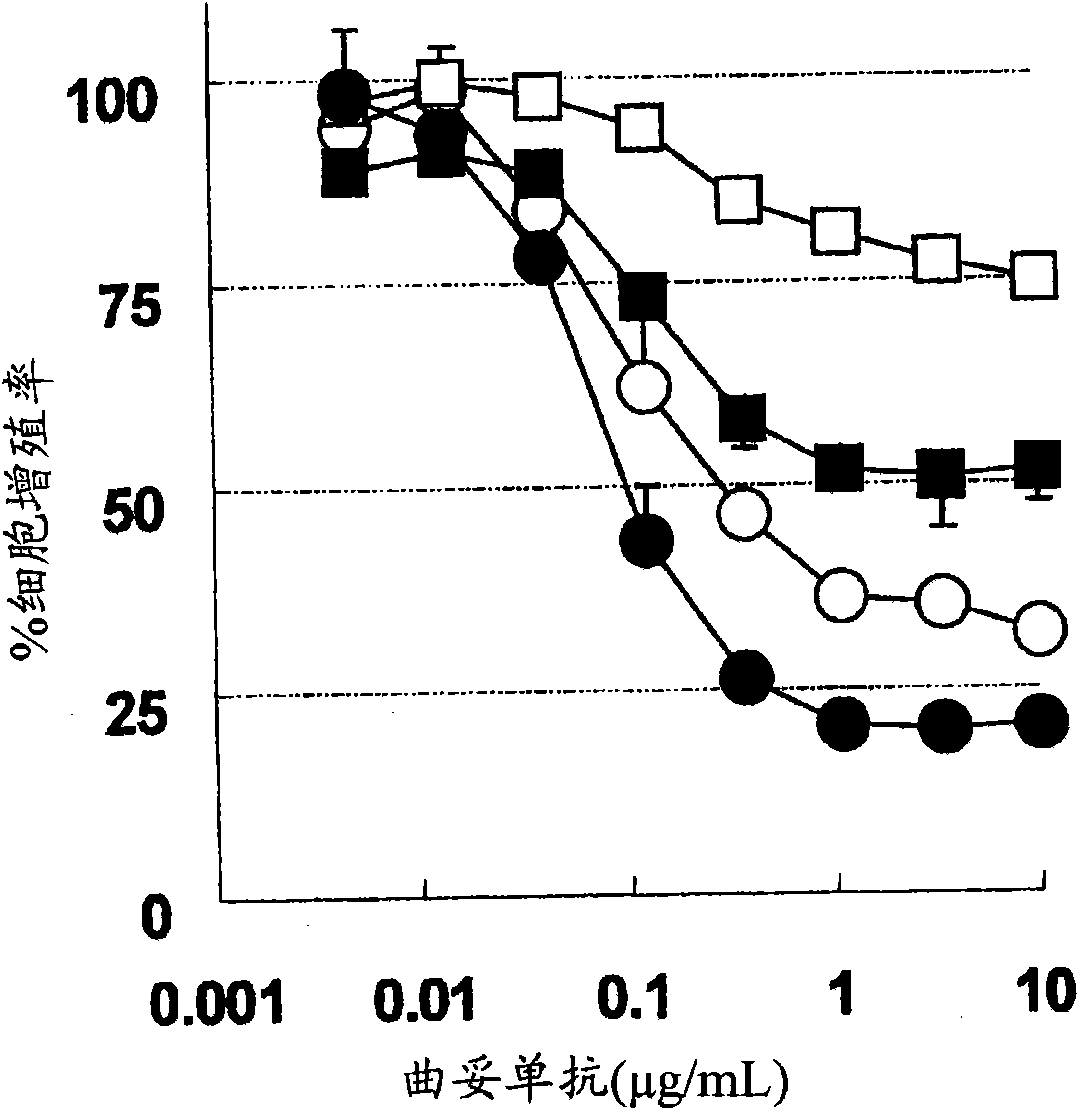
[1297] BT474 cells with high sensitivity to trastuzumab (American Type Culture Collection, HTB-20, J Natl Cancer Inst 61:967-978 (1978)), and BT-474 cells with low sensitivity to trastuzumab were used , the BT-474 cells with low sensitivity to trastuzumab were cultured for 3 months by culturing the BT474 cells with high sensitivity to trastuzumab in RPMI complete medium containing 5 μg / mL trastuzumab or longer. The cofilin 1 gene was knocked down by the following RNAi method. Three kinds of Stealth RNAi corresponding to cofilin 1 gene (CFL Stealth Select 3 RNAi, Invitrogen Company, HSS141559: HSS141560: HSS141561) and LipofectaminRNAiMAX (Invtrogen Company, 13778-150) were reduced in serum in Opti-MEM(R)I Mix in culture medium (Invitrogen, 31985-070) to 36.7nM x3 (total 110nM) and 11μL / mL. After standing at room temperature for 20 minutes, the Stealth RNAi was mixed with each BT-474 cell suspension adjusted to 22,000 cells / mL in the culture medium at a liquid volume ratio of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com