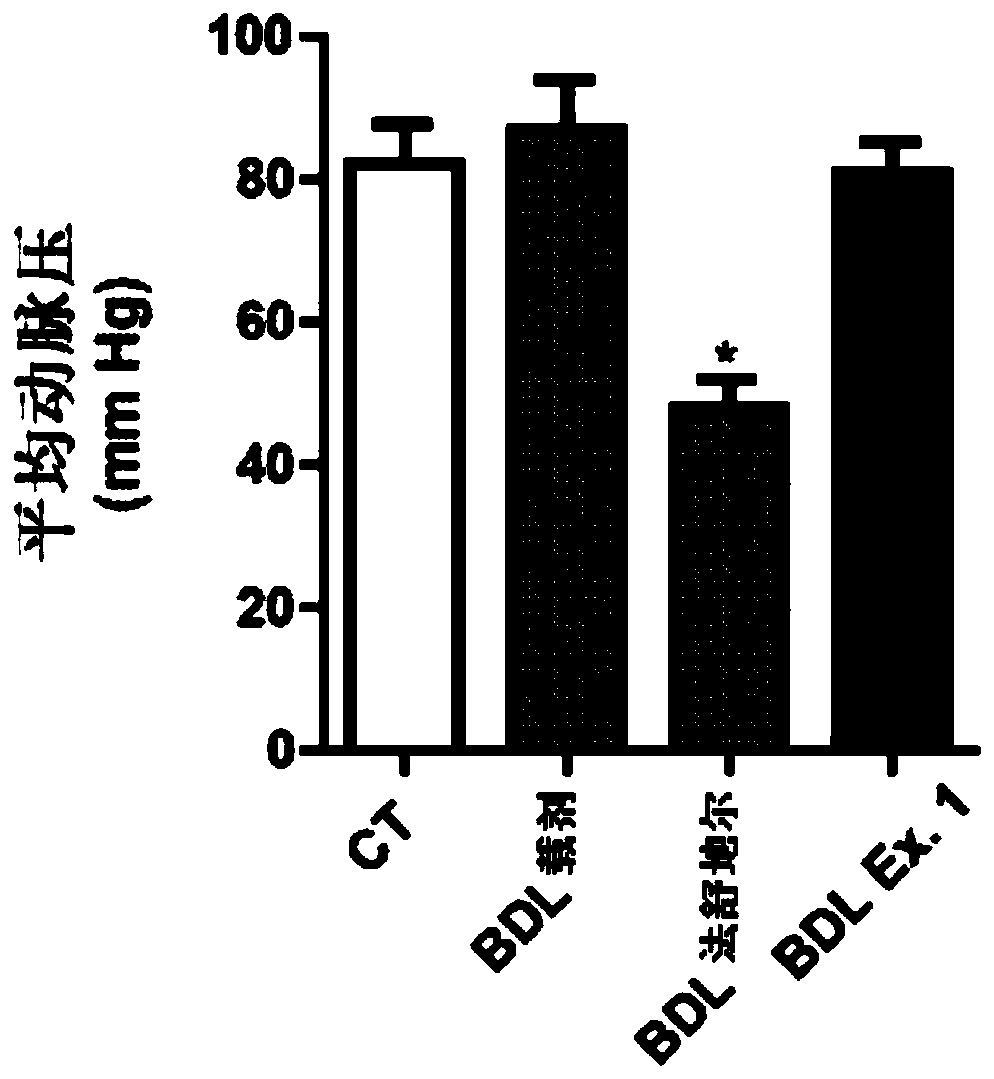
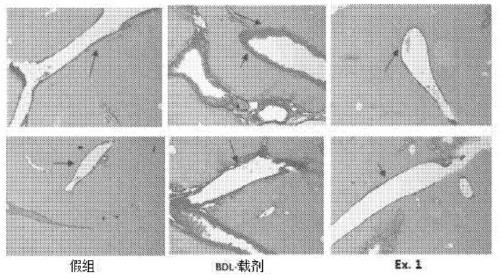
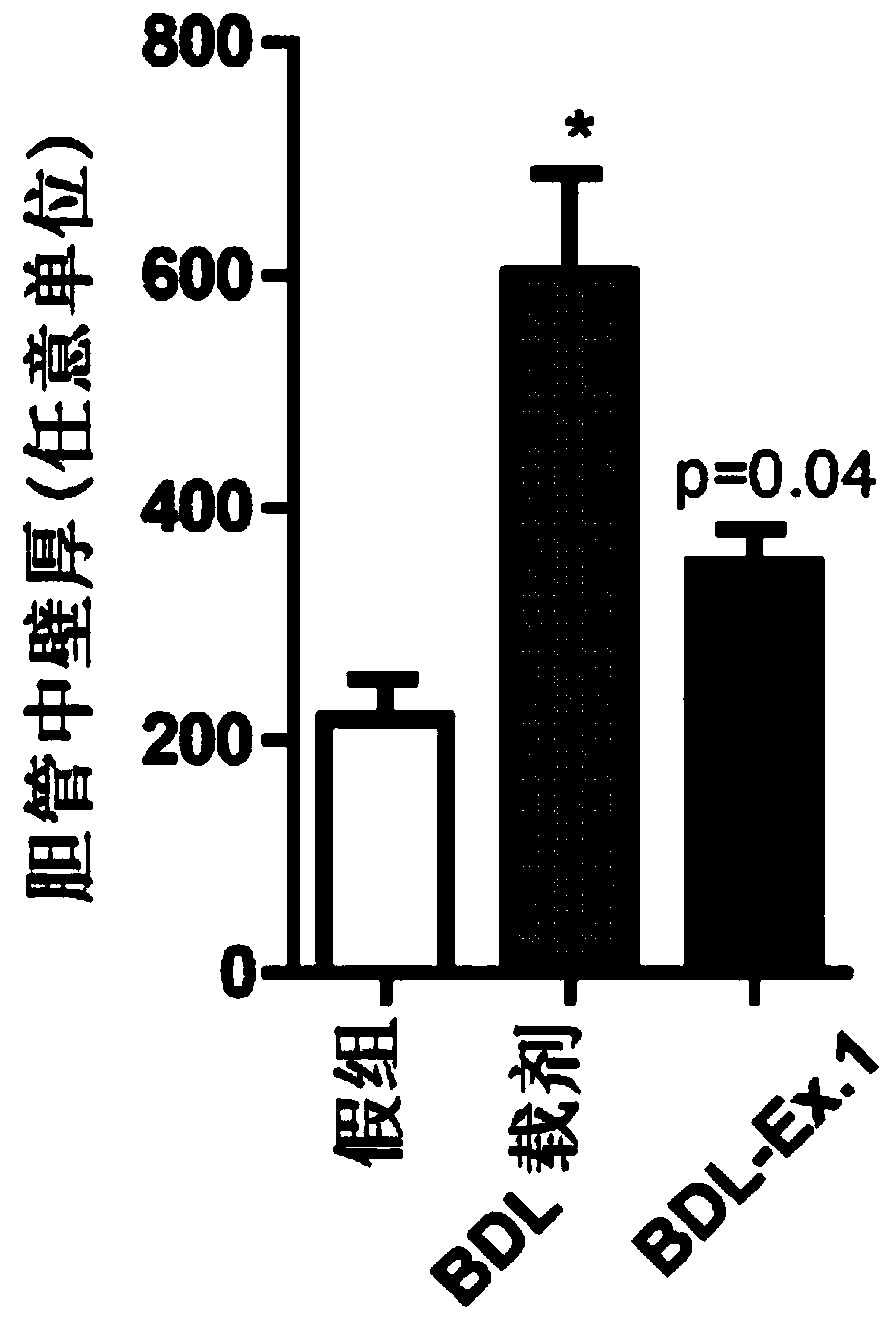
Vinylheterocycles as RHO-associated coiled-coil kinase (ROCK) inhibitors
A heterocyclic group and group technology, applied in the field of vinyl heterocyclic rings as RHO-related coiled-coil kinase (ROCK) inhibitors, can solve the problem that the use has not yet been approved in the United States
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0288] Example 1. (E)-5-methoxy-2-(4-(2-(pyridin-4-yl)vinyl)-[2,4'-bipyrimidinyl]-2'-yl)isoindyl Indoline (Ex.1).
[0289]
[0290] Step 1: 2-(5-Methoxyisoindolin-2-yl)pyrimidine-4-carbonitrile (1-3): To 2-chloropyrimidine (1-1, 1.5g, 10.8mmol) and 5 - A stirred mixture of methoxyisoindoline hydrochloride (1-2, 2.0 g, 10.8 mmol) in aqueous acetonitrile (40 mL) was added dropwise with N,N-diisopropylethylamine (4.14 mL, 23.76 mmol). The reaction mixture was stirred at 80°C for 3 hours. The resulting solution was concentrated under vacuum then triturated with water and filtered. The filter cake was thoroughly washed with water and dried under vacuum to obtain brown product 1-3 (2.45 g, yield: 90%). MS (ESI + ):m / z:253.1(M+H) + .
[0291] Step 2: 2-(5-methoxyisoindoline-2-yl) pyrimidine-4-formimidate (carbimidate) methyl ester (1-4): at 0 ° C, to 1-3 (1.2 g, 4.8 mmol) in anhydrous dichloromethane (25 mL) was added acetyl chloride (3.4 mL, 47.6 mmol) followed by anhydro...
Embodiment 2
[0295] Example 2. (E)-2-(4-(2-(1H-pyrazol-3-yl)vinyl)-[2,4'-bipyrimidinyl]-2'-yl)-5-methoxy Isoindoline (Ex.2).
[0296]
[0297] Step 1: 1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazole-4-carbaldehyde (2-3):
[0298] To a stirred solution of pyrazolecarbaldehyde (2-1,300 mg, 3.1 mmol) in tetrahydrofuran was added 3,4-dihydro-2H-pyran (2-2,867 mg, 10.3 mmol) followed by a catalytic amount of trifluoroacetic acid. The resulting solution was refluxed for 4 hours, then cooled to room temperature. The reaction was quenched by adding a trace of sodium hydride. The solvent was removed under vacuum and the residue was purified by silica gel column chromatography to afford 2-3 (520 mg, 92%). MS (ESI + ):m / z:181.1(M+H) + .
[0299] Step 2: (E)-5-Methoxy-2-(4-(2-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-3-yl)ethenyl) -[2,4'-bipyrimidine]-2'-yl)isoindoline (2-4):
[0300] To a stirred solution of 1-7 (50 mg, 0.15 mmol) and tetrabutylammonium bromide (TBAB, 40 mg, 0.12 mmol) in 3 mL of ...
Embodiment 3
[0303] Example 3. (E)-4-(2-(2'-(5-methoxyisoindolin-2-yl)-[2,4'-bipyrimidin]-4-yl)ethenyl) Quinoline (Ex.3)
[0304]
[0305] -Preparation by following the same procedure as described in step 5 of Example 1. A yellow solid was obtained in 56% yield. 1 H-NMR (300MHz, CDCl 3 ): δ (ppm): 9.03 (d, J = 5.1Hz, 1H), 8.99 (d, J = 4.3Hz, 1H), 8.84 (d, J = 15.9Hz, 1H), 8.67 (d, J = 4.8 Hz, 1H), 8.32(d, J=8.1Hz, 1H), 8.22(d, J=8.1Hz, 1H), 7.75(m, 4H), 7.48(d, J=5.1Hz, 1H), 7.41( d, J=15.6Hz, 1H), 7.26(s, 1H), 6.90(s, 1H), 6.87(d, J=2.4Hz, 1H), 5.06(br, 4H), 3.85(s, 3H). MS (ESI + ): m / z: 459.3 (M+H) + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com