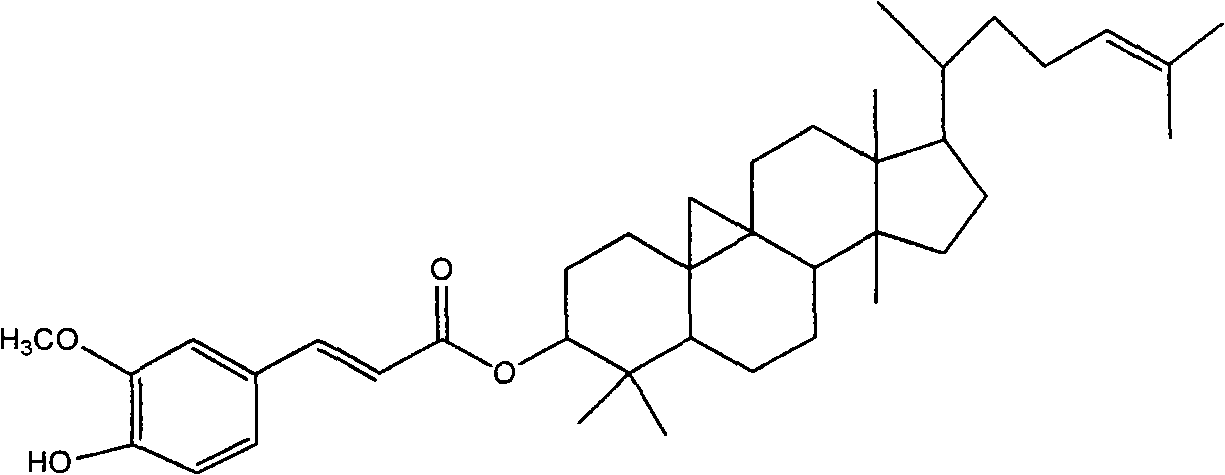
Composition preparation and purification process of cycloartenyl ferulate
A technology of pineapple enol ferulate and pineapple alcohol ferulate, which is applied in the field of composition preparation and purification process of pineapple enol ferulate, and achieves fewer unit operations, higher yield and fewer steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Get 1 kg of oryzanol containing about 40% cycloarneol ferulate, heat and dissolve with 15 kg of ethyl acetate, leave it at room temperature until crystals are precipitated, filter, and dry. Repeat the above process for 10 times with the dried crystals to obtain a reference substance with a content of cycloartenol ferulic acid ester of more than 99%.
Embodiment 2
[0035] 1. Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; methanol-acetonitrile (40:60) is used as mobile phase; flow rate is 1.5ml / min; detection wavelength is 327nm; sensitivity is 0.005 AUFS. The number of theoretical plates should not be less than 2000 according to the calculation.
[0036] 2. Preparation of the test solution: Accurately weigh 10 mg of the commercially available oryzanol raw material and 10 mg of the present invention, put them in a 10 ml measuring bottle, add water to dissolve and constant volume, and shake well to obtain the test solution.
[0037] 3. Determination: Accurately draw 10 μl of the test solution, inject it into a liquid chromatograph, record the chromatogram, and check the percentage content of each component by the area normalization method.
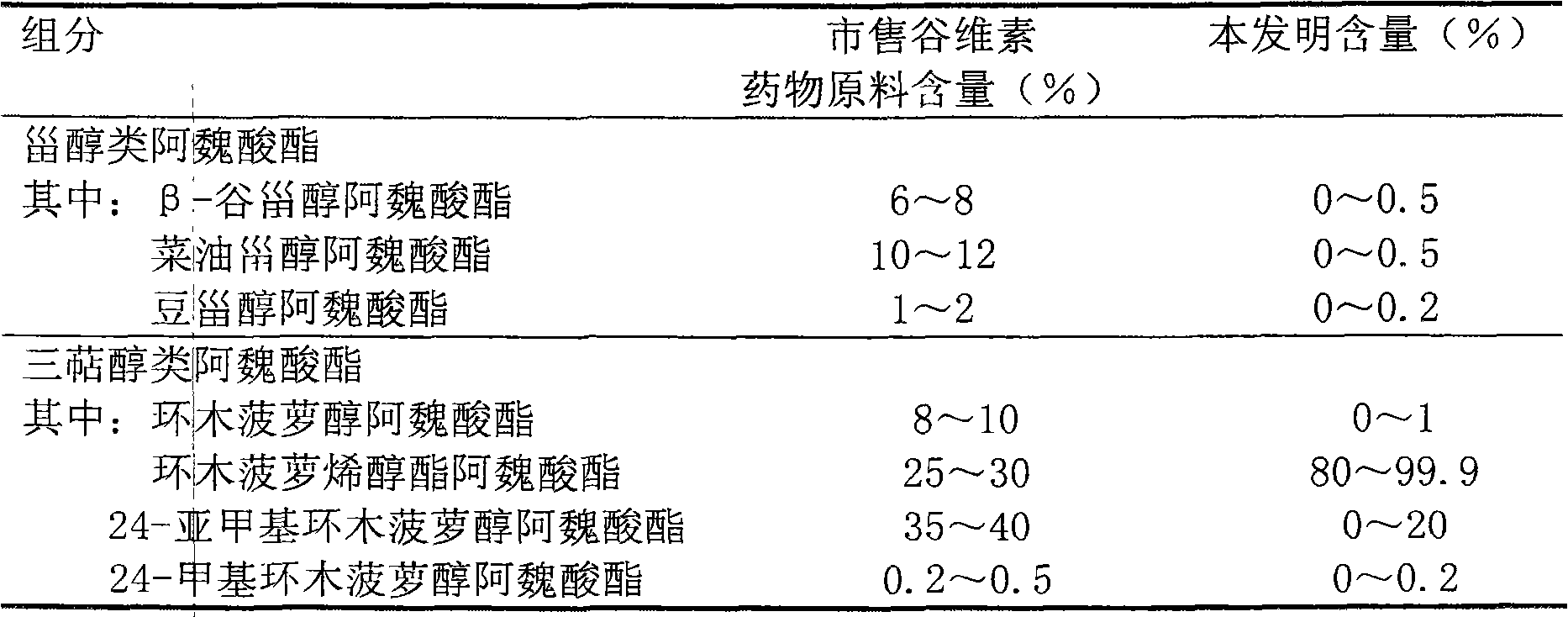
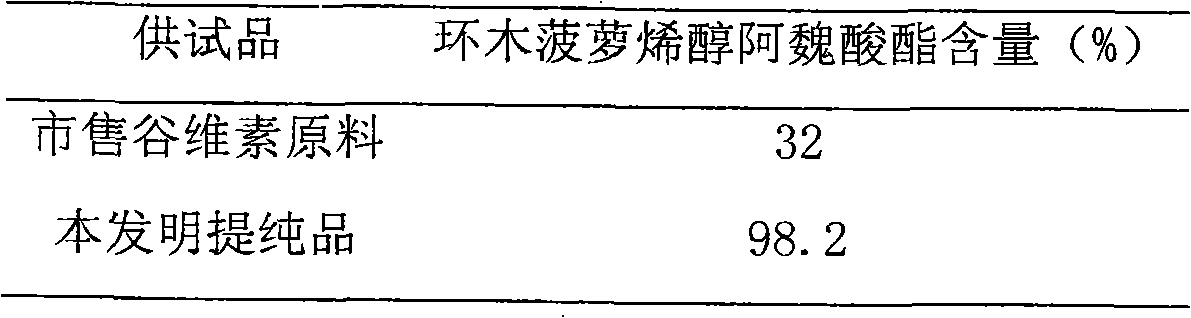
[0038] 4. Measurement results
[0039] Table 2 Measurement results
[0040]
[0041] Illustrate that technique of the present inventi...
Embodiment 3
[0043] prescription
[0044] 1000 bottles of freeze-dried powder of the present invention for injection (containing 18mg / bottle of the present invention):
[0045] Purified product of the present invention 18g
[0046] Macrogol 400 250g
[0047] Tween 80 250g
[0048] 10% Mannitol 6000g
[0049]
[0050] In the prescription, the raw material of the present invention is used as the main agent, Tween 80 is used as a solubilizing agent, polyethylene glycol 400 is used as a cosolvent, mannitol is used as a proppant, and water for injection is used as a freeze-drying solvent.
[0051] Preparation process
[0052] Take by weighing the purified product of the present invention, Tween-80, PEG-400, mannitol and 80% of the prescription amount of water for injection, stir to make it dissolve until the solution is clear, stir at room temperature for 20 minutes, and filter the mixture with a 0.45 μm microporous membrane Filtrate, add water for injection to the filtrate to the full ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com