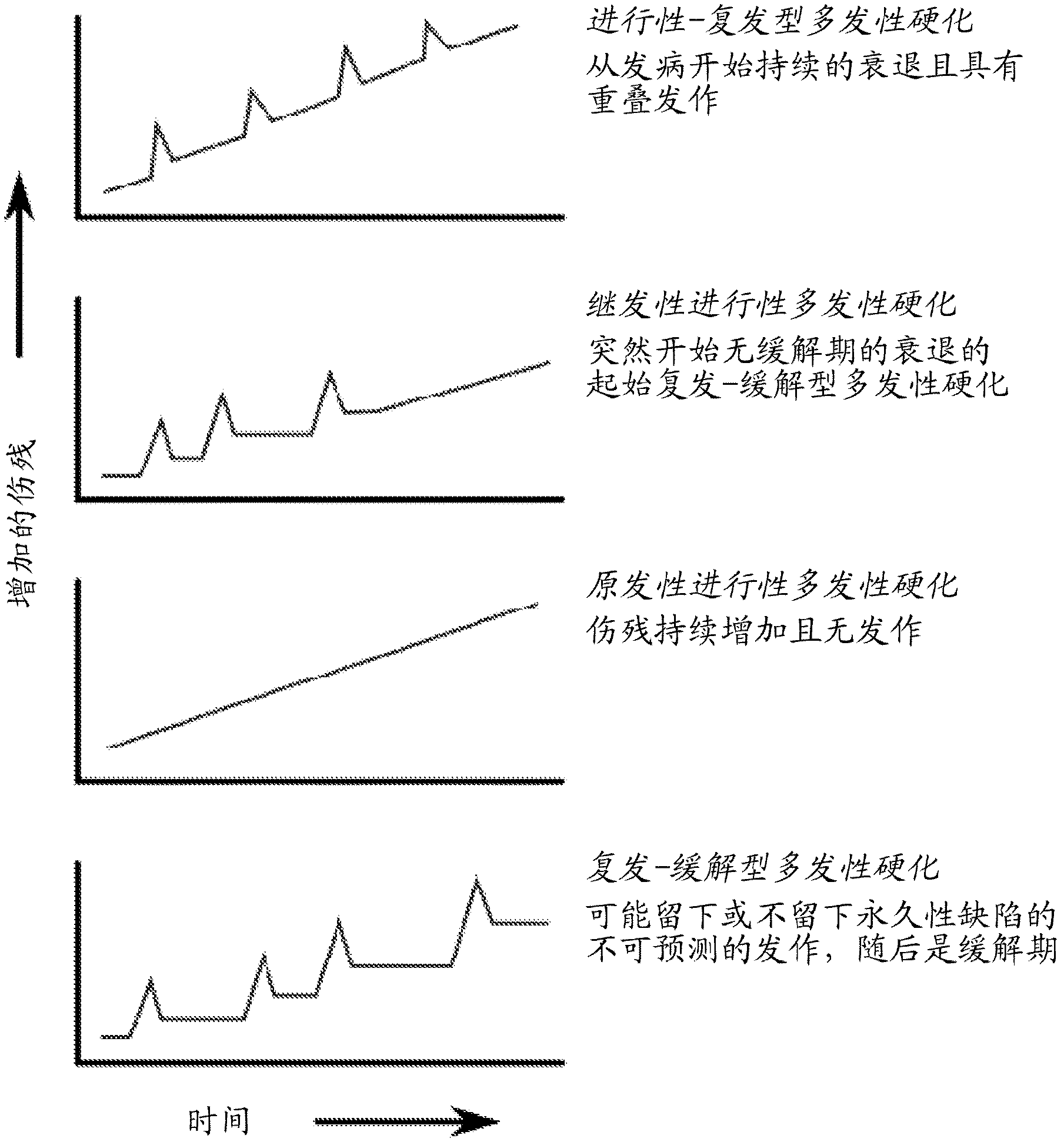
Treatment for multiple sclerosis
A multiple sclerosis, subject technology, applied in chemical instruments and methods, anti-animal/human immunoglobulin, antibodies, etc., can solve the problem of long-term recovery of patients without significant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0270] Example 1 : Exemplary antibodies and animals used in the present invention
[0271] MOR-GM is used in the present invention as an exemplary GM-CSF antagonist. MOR-GM is a fully human GM-CSF-specific antibody (WO 06 / 122797). The heavy chain variable region of MOR-GM is shown in SEQ ID No.:3, and the light chain variable region is shown in SEQ ID No.:4.
[0272] Antibody 22E9, an anti-mouse GM-CSF antibody, was used in other experiments (AbD Serotec, Martinsried / Germany; Cat. No. 1023501).
[0273] Obviously, any other GM-CSF antagonist, eg any antibody comprising an amino acid fragment selected from SEQ ID No.: 1-45 may be used according to the invention.
[0274] Male Dark Agouti rats, 7-8 weeks old (Harlan Laboratories, Inc., Indianapolis / IN) were housed under clean conventional conditions at 21 ± 3°C, 40-70% relative humidity and a 12-hour light / dark cycle. Rats were housed in pairs and had free access to rodent chow (SSNIFF, Bio-Services, The Netherlands). Indi...
Embodiment 2
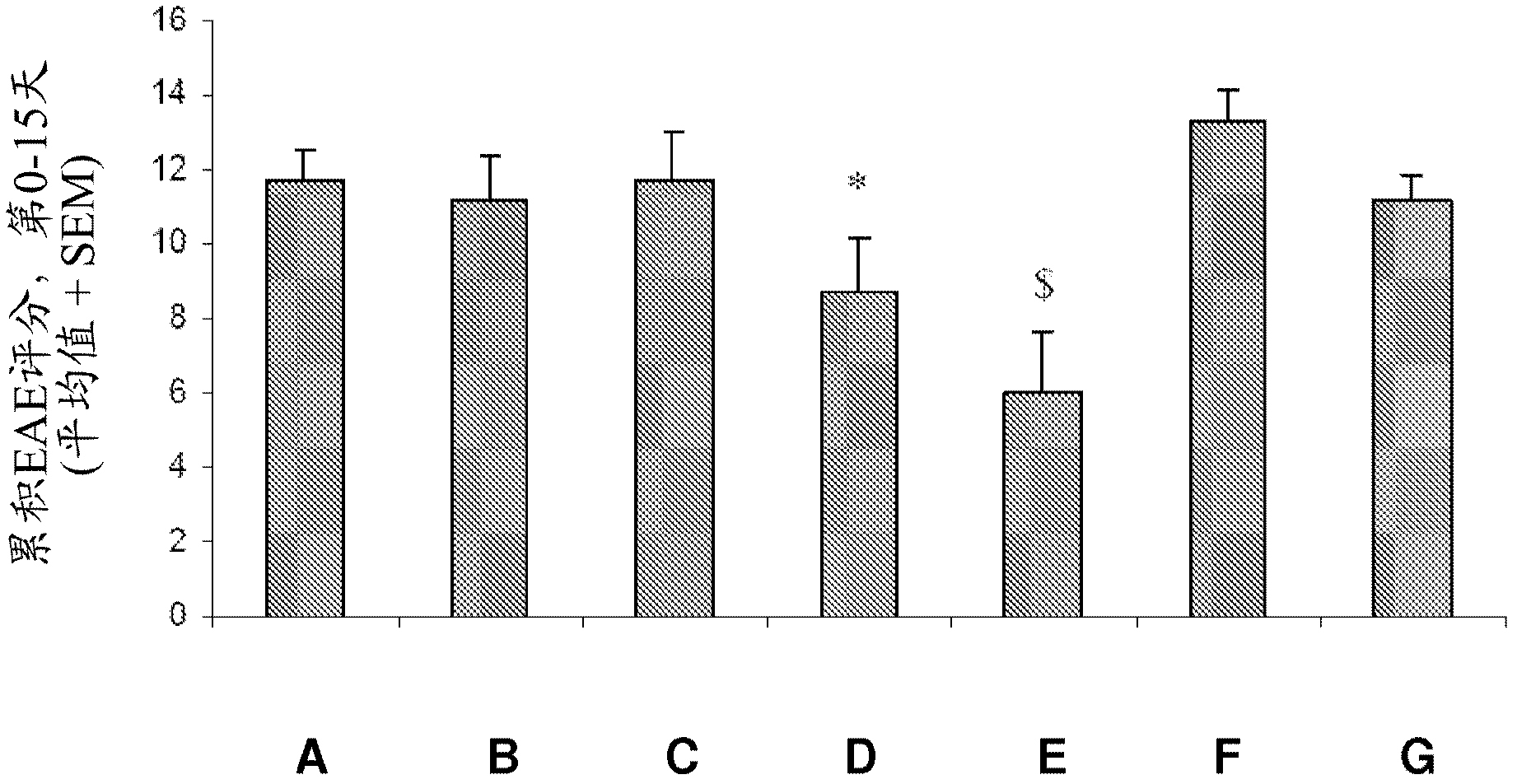
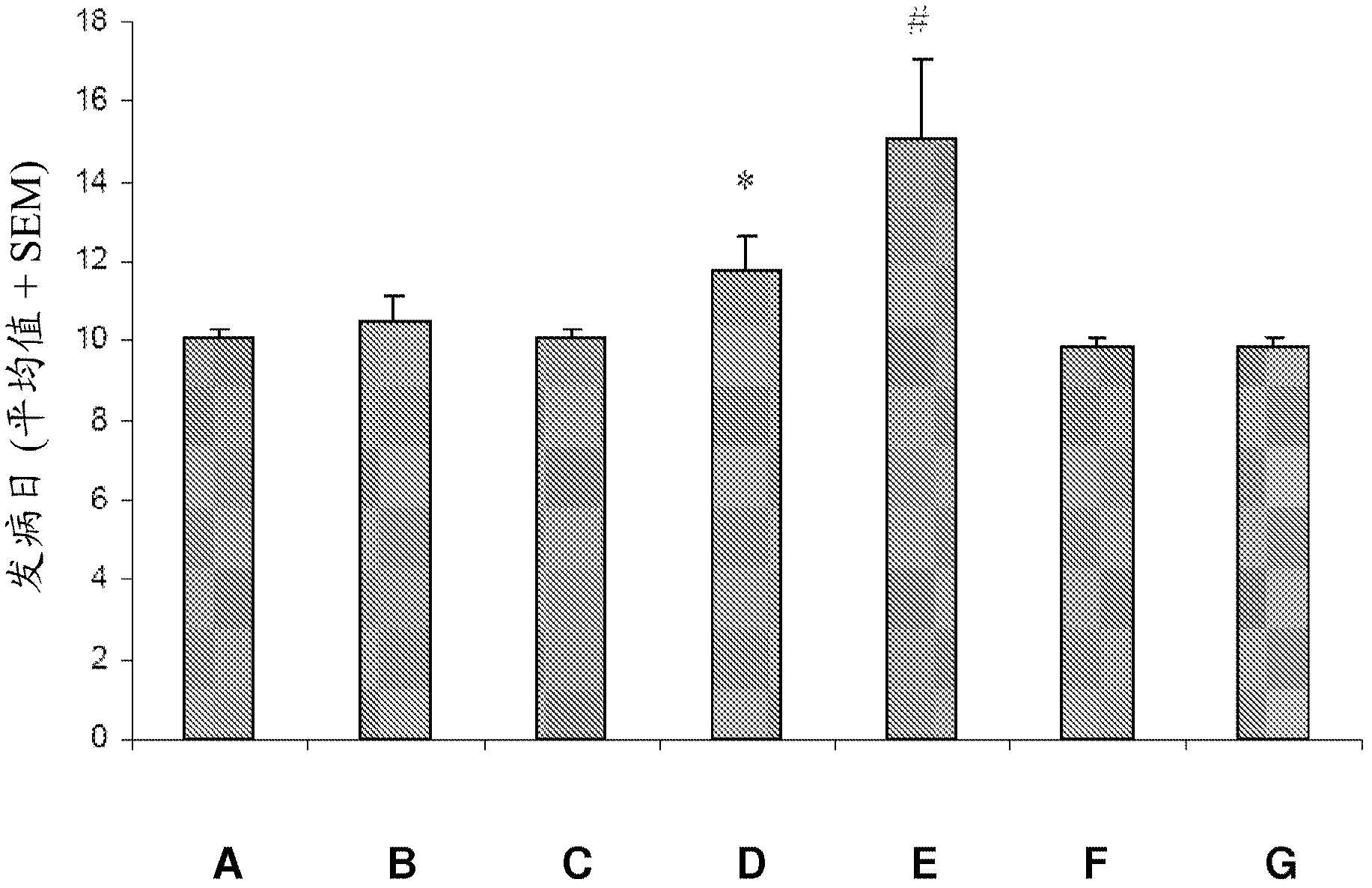
[0275] Example 2 : Therapeutic effectiveness of GM-CSF antagonists in the EAE model of MOG-induced MS
[0276] For the induction of experimental autoimmune encephalomyelitis (EAE), two sites on both sides of the dorsal base of the tail were treated with incomplete Freund's adjuvant (IFA) in 200 μl and 10 nM NaAc pH 3.0. Male DA rats were immunized by intradermal injection of 15 μg of recombinant myelin-oligodendrocyte-glycoprotein (rMOG) emulsified in a 1:1 mixture. To assist immunity, inhalation of oxygen and N with 2-4% isoflurane 2 O mixture to anesthetize rats.
[0277] The effect of intraperitoneal administration of the test compound MOR-GM was examined compared to vehicle (PBS) treatment and compared to the group treated with the non-specific / irrelevant isotype control antibody MOR-NOGM (50 mg / kg). Prophylactic treatment with compound MOR-GM was tested at 3 doses, namely 10 mg / kg, 20 mg / kg and 50 mg / kg. Compounds were administered on days 7, 10, 14, 17 and 21. Also...
Embodiment 3
[0311] Example 3 : Therapeutic effectiveness of GM-CSF specific antibodies comprising SEQ ID NO.3 or 4
[0312] Example 2 was repeated. A GM-CSF-specific antibody comprising the amino acid sequence of the heavy chain variable region as depicted in SEQ ID No.: 1 or comprising the amino acid sequence of the light chain variable region as depicted in SEQ ID No.: 2 was used As GM-CSF antagonist. Species other than mice may be used, especially species with which the antibodies used in this experiment cross-react. Preferably the animal species used in this experiment is rat.
[0313] Animals, such as rats, treated with an isotype control antibody, are compared to those receiving an amino acid sequence comprising a heavy chain variable region as depicted in SEQ ID No.: 1 or comprising a light chain variable region as depicted in SEQ ID No.: 2. Animals with GM-CSF-specific antibodies to the amino acid sequence of the chain variable region showed a significant increase in the sign...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com