Antibody compositions with altered fab sialylation
A sialylation and antibody technology, applied in the fields of antibody, drug combination, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0042] The term "antibody" as used throughout the present invention encompasses, for example, polyclonal or monoclonal antibodies. The term "antibody" also includes derivatives or fragments thereof that retain antigen-binding specificity. Thus, embodiments such as chimeric (human constant regions, non-human variable domains), single chain and humanized (human antibodies with non-human CDRs) antibodies are also included, as well as antibody fragments, e.g., particularly , Fab or Fab' fragment. Antibody fragments or derivatives also include Fd, F(ab') 2 , Fv or scFv fragments; see, e.g., Harlow and Lane "Antibodies, A Laboratory Manual", Cold Spring Harbor Laboratory Press, 1988 and Harlow and Lane "Using Antibodies: A Laboratory Manual", Cold Spring Harbor Laboratory Press, 1999.
[0043] The term "Fab region" as used throughout the present invention refers to the region of an antibody consisting of one constant and one variable domain from each of the heavy and light chains ...
Embodiment 1
[0122] [Example 1: Fractionation of IVIG using 2,6-sialic acid-specific Sambucus nigra lectin (SNA)]
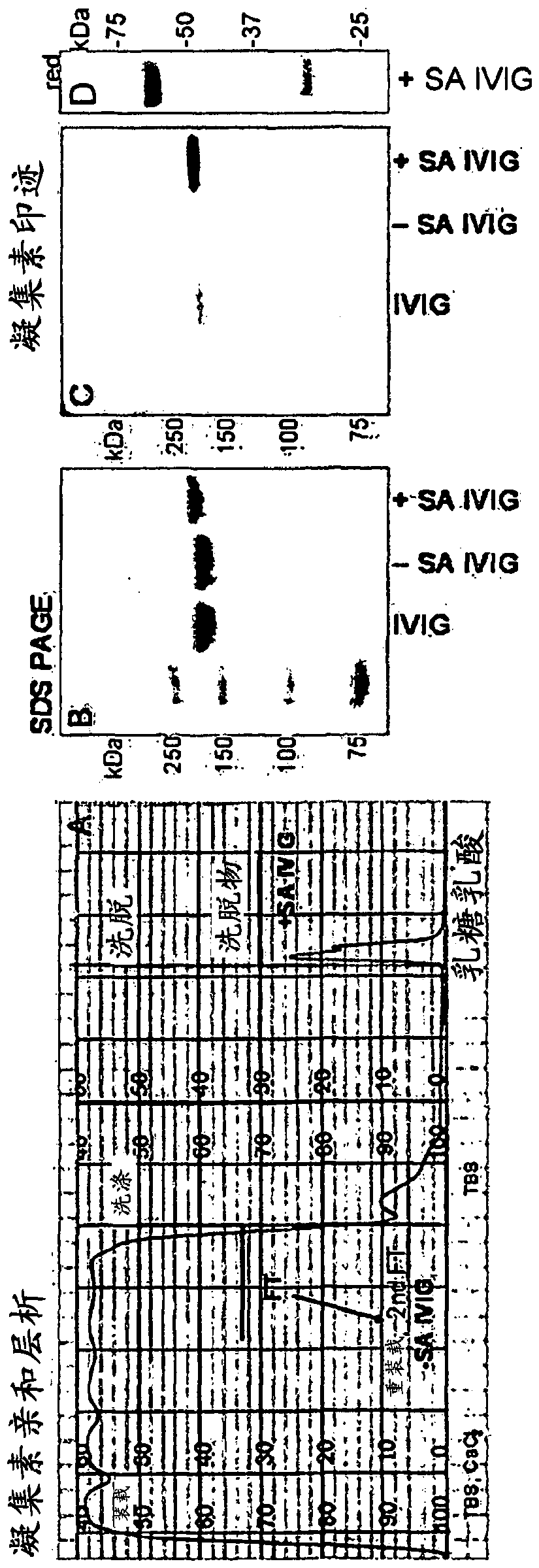
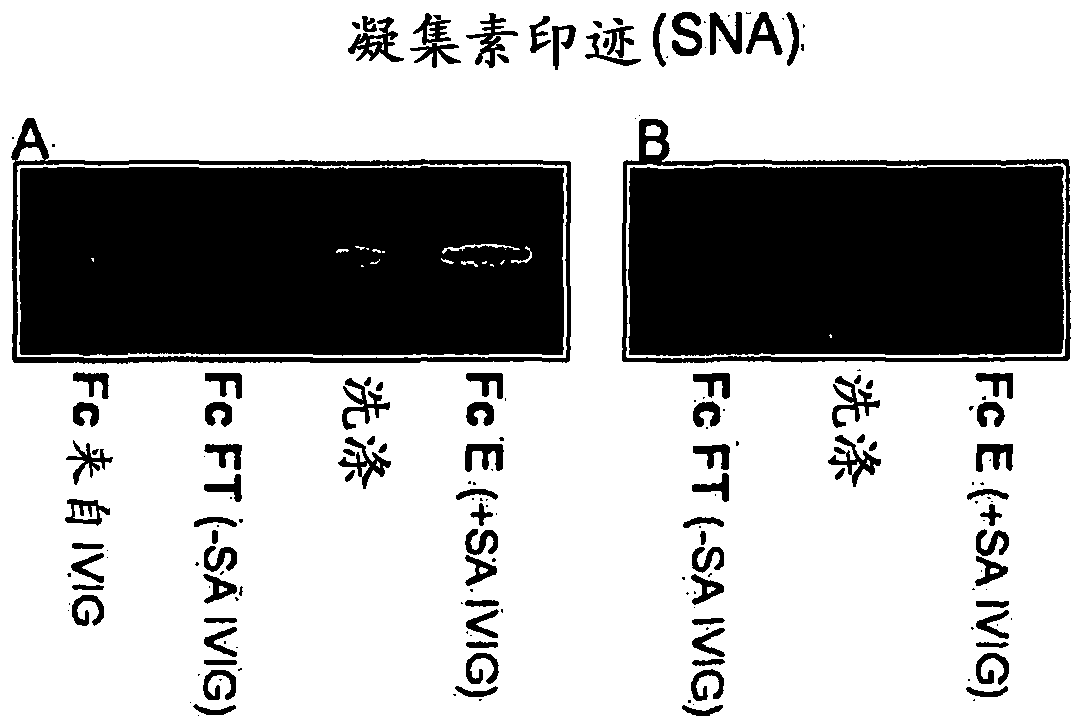
[0123] 1 g IVIG in 100 ml tris-buffered saline (TBS), 0.1 mM CaCl at pH 9-10 2 Recirculate for 1 h on a 100 ml Sambucus nigra lectin (SNA) column. Flow through with collection 100ml TBS / CaCl 2washing. After washing with 2 x 200ml of TBS, the fraction bound to the SNA column (+SA IVIG) was eluted with 200ml of 0.5M lactose in 0.2M acetic acid. Run a 2nd flow-through fraction on the column to obtain -SA IVIG ( figure 1 ).
[0124] Total sialic acid content in IgG was monitored by SDS-PAGE (non-reducing conditions) and lectin blot ( figure 1 ). The resulting IVIG fractions were separated by SDS-PAGE using Nupage 10% BisTris gel. Gels were stained with Coomassie and blotted to nitrocellulose. Blots were probed with biotin-SNA and AP-streptavidin and visualized with chromogenic substrates. exist figure 1 In D, heavy and light chains were separated by SDS-PAGE under reduc...
Embodiment 2
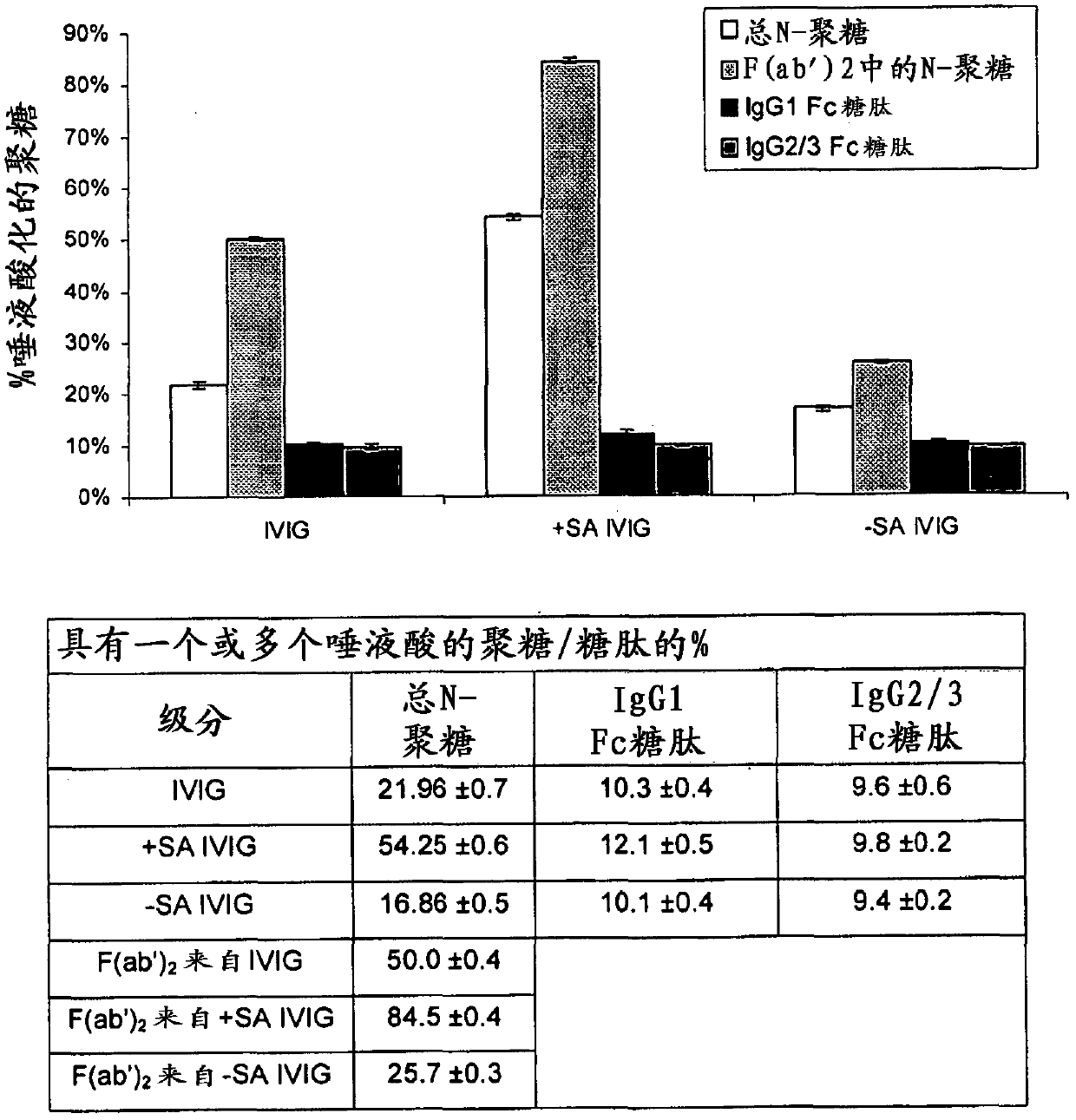
[0128] [Example 2: Glycan analysis]
[0129] To study the glycosylation profile of IVIG before and after isolation, identify and characterize tryptic glycopeptides derived from the Fc region of typical IVIG preparations, and characterize the total N-glycan population of IVIG by glycan release and analysis.
[0130] 【method】
[0131] [(a) Determination of IgG1 and IgG2 / 3 Fc Glycan Characterization by Peptide Localization]
[0132] IVIG samples were analyzed by liquid chromatography-mass spectrometry (LC-MS) after trypsinization to determine glycan profiles specific to IgG1 and IgG2 / 3 Fc regions. Glycopeptides that monitor the IgG2 Fc region are also found in the IgG3 Fc region, hence they are referred to as IgG2 / 3. Based on the known relative abundance of IgG subclasses in serum, most of the signal seen for IgG2 / 3 peptides was expected to originate from IgG2 molecules. Denaturation, reduction (heating with guanidinium-HCl and DTT) and alkylation (with iodoacetamide) followed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com