Compounds for the treatment/prevention of ocular inflammatory diseases
A technology of inflammatory diseases and compounds, applied in sensory diseases, anti-inflammatory agents, organic chemistry, etc., can solve problems such as inability to be fully accepted by subjects, irritation of oil excipients, painful vision, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0118] These compositions may be prepared by any of the methods of preparing dosage forms well known in the art of pharmacy.
[0119] According to another aspect, the compounds of the present invention may be combined or used in combination with other therapeutic agents. For example, one or more compounds of the present invention or a pharmaceutically acceptable salt thereof, in particular trepelimus and / or animolimus, may be used together with other traditional medicines for the treatment of ocular inflammatory diseases , so as to treat the subject. Multiple active substances may be administered simultaneously, consecutively or over a period of time. The compound of the present invention or a pharmaceutically acceptable salt thereof will preferably not be administered in combination with an Lck enzyme inhibitor.
[0120] According to one embodiment, the present invention thus relates to pharmaceutical compositions comprising, as active substance, at least one compound of fo...
Embodiment 1
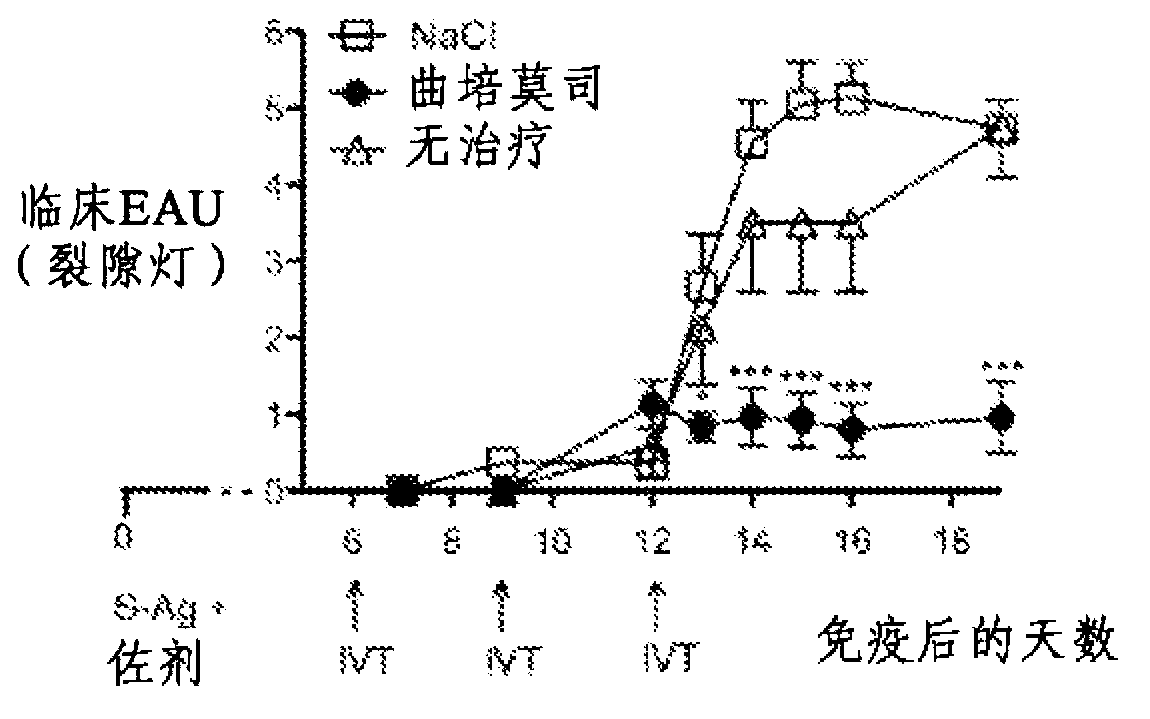
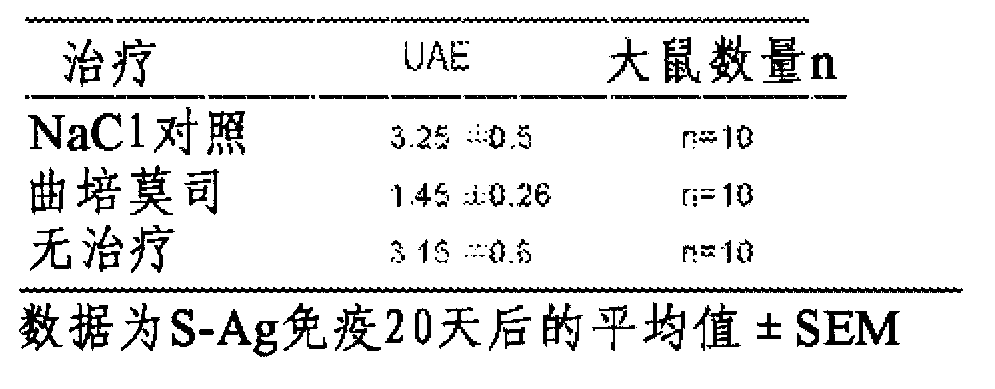
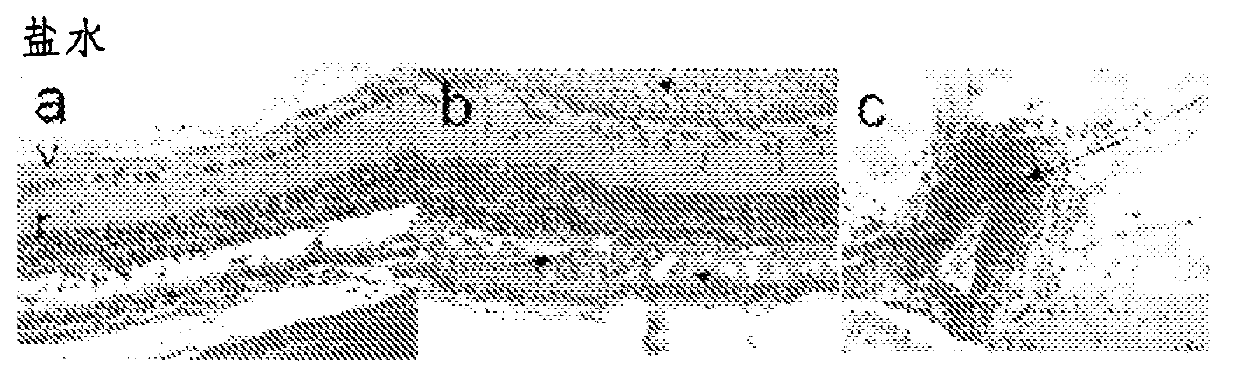
[0127] Example 1: Uveitis
[0128] The eye is a site of immunological privilege; however, eye disease caused by an imbalance in the immune system can develop and result in visual impairment that can lead to blindness. Animal models of primarily experimental autoimmune uveitis (EAU) and endotoxin-induced uveitis (EIU) are considered relevant clinical models of ocular disease and are invaluable for studying immune mechanisms capable of modulating disease in humans. tool:
[0129] EAU induced by immunization with purified rat retinal antigens, mainly S-antigen (S-Ag), is considered to be a relevant model for the study of the mechanism of human posterior uveitis and the development of new therapeutic strategies for uveitis;
[0130] EIU is a model of acute uveitis, which is spontaneously resolved by the natural immune system. This model is a valid model for studying local aspects of ocular inflammation and is considered a relevant model for anterior uveitis in humans.
[0131] ...
Embodiment 2
[0204] Example 2: Dry Eye Syndrome
[0205] Existing therapies are essentially palliative and aim to replace or preserve the subject's tears through the frequent administration of artificial tears. Severe dry eye is characterized by severe corneal damage with an increased risk of secondary infection and is sometimes treated with anti-inflammatory therapy.
[0206] Several animal models have been developed to reflect the different pathophysiological mechanisms involved in KCS. studied the effect of trepelimus in a mouse model of dry eye; this model uses pharmacological inhibition of tear production to induce changes in the ocular surface epithelium similar to human KCS, which are exacerbated by drying environmental stress.
[0207] Dry eye was induced in mice by combining scopolamine (which blocks muscarinic cholinergic receptors) and placing mice in an extractor hood with reduced humidity and increased airflow disease. Aqueous tear production and volume, tear clearance and ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap