A kind of 4-(1-substituted phenylvinyl) biphenyl derivative and its preparation method and application
A technology of phenylvinyl and derivatives, applied in the field of medicine, can solve the problems of single tumor inhibitory spectrum and prone to drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
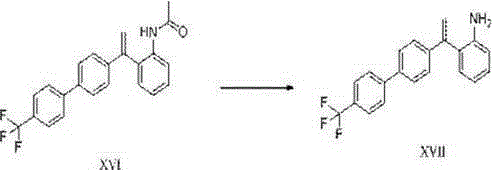
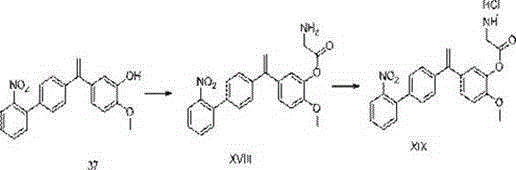
Image
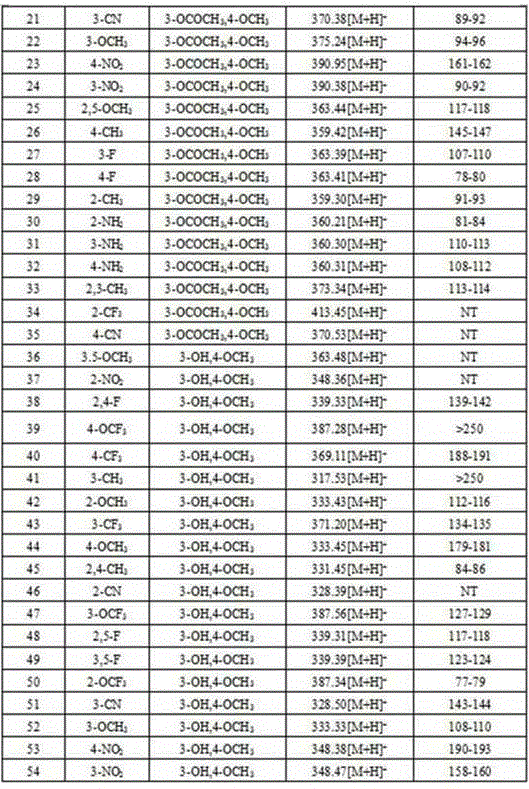
Examples
Embodiment 14
[0066] Preparation of Example 14'-(1-(4-methoxyphenyl)vinyl)-2-nitrobiphenyl (compound 1 in Table 1)
[0067]
[0068] (1) Preparation of intermediate: 2-nitro-4'-acetyl-1,1'-biphenyl (Ⅱ)
[0069] p-Acetophenoneboronic acid (0.23g, 1.4mmol), 2-nitrobromobenzene (I) (0.20g, 1mmol), cesium carbonate (0.489g, 1.5mmol) dissolved in water (0.75ml) and dioxane ( 2.2ml), then added bis(triphenylphosphine)palladium chloride (0.02g, 0.03mmol), under nitrogen protection, refluxed and stirred at 100°C for 1hr, the reaction solution was a brown clear liquid. The reaction solution was slowly added to crushed ice (400g), then stirred for 1h. Add 15ml of water, extract with ethyl acetate (10ml×3), combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, and separate by column chromatography (PE:EtOAc=20:1) to obtain a white solid, Yield 86%. Obtain intermediate (II);
[0070] (2) Preparation of intermediate: 2-nitro-4'-acetyl-1,1'-bip...
Embodiment 2-7
[0075] Repeat Example 1, the difference is: using different raw materials, thereby preparing compound 2-7. details as follows:
[0076] 3-methyl bromobenzene, 2-methoxy bromobenzene, 3-methoxy bromobenzene, 4-nitrobromobenzene, 3-nitrobromobenzene, 2-methyl bromobenzene and p-acetylphenylboronic acid respectively Prepare the corresponding substituted p-acetylbiphenyl, and then make the corresponding substituted 4'-acetyl-1,1'-biphenyl p-toluenesulfonylhydrazone with p-toluenesulfonyl hydrazide, and finally mix with p-methoxy bromide Benzene reacts to generate compounds 2, 3, 4, 5, 6, and 7 in Table 1.
Embodiment 8
[0077] Example 8: Preparation of 4'-(1-(3-acetoxy-4-methoxyphenyl)vinyl)-3,5-dimethoxybiphenyl (compound 8 in Table 1)
[0078]
[0079] (1) Preparation of intermediate: 3,5-dimethoxy-4'-acetyl-1,1'-biphenyl (VII)
[0080] p-Acetophenoneboronic acid (0.23g, 1.4mmol), 3,5-dimethoxybromobenzene (VI) (0.22g, 1mmol), cesium carbonate (0.489g, 1.5mmol) were dissolved in water (0.75ml) and di Oxycycline (2.2ml) and bis(triphenylphosphine)palladium chloride (0.02g, 0.03mmol) were added, under nitrogen protection, and stirred at reflux at 100°C for 1hr, the reaction solution was a brown clear liquid. The reaction solution was slowly added to crushed ice (400g), then stirred for 1h. Add 15ml of water, extract with ethyl acetate (10ml×3), combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, and separate by column chromatography (PE:EtOAc=20:1) to obtain a white solid, Yield 86%. Intermediate (VII) is obtained;
[0081] (2) P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com