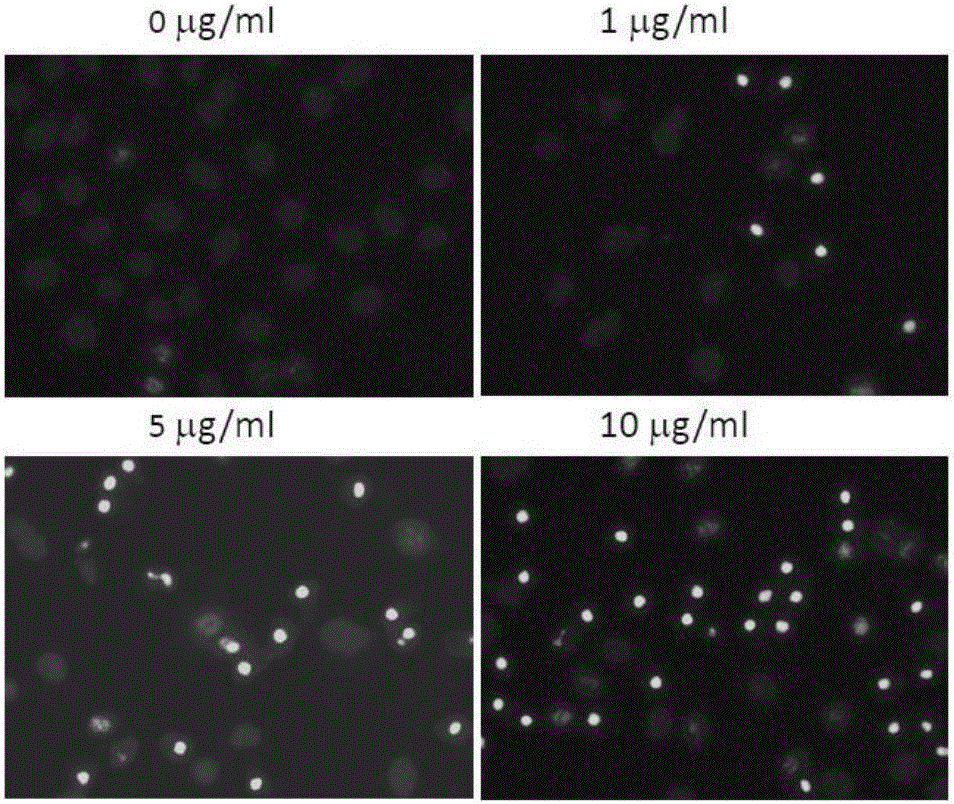
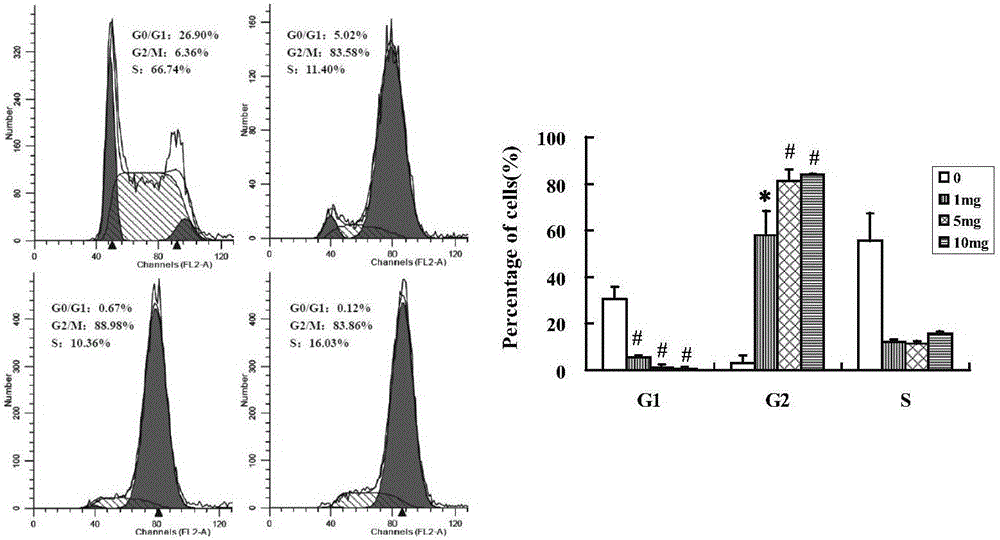
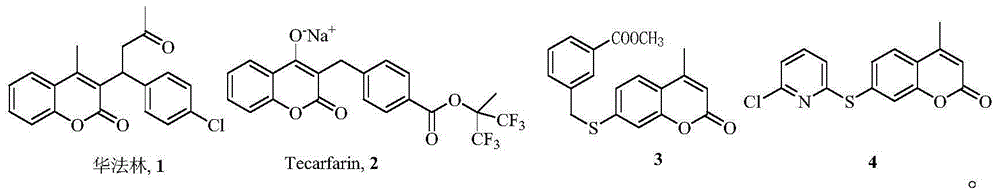
7-oxygen, sulphur or aza-substituent coumarin and derivative and application thereof
A technology of coumarin derivatives and nitrogen substitution, applied in drug combination, organic chemistry, anti-tumor drugs, etc., can solve the problems of rare and few anti-tumor active clinical drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesize compound 7-oxygen, sulfur or nitrogen substituted coumarin and derivatives thereof of formula 8 or 12 according to general formula one or general formula two,
[0029] 1. according to general formula one, with commercially available 7-hydroxyl, mercapto or amino-substituted coumarin (5) and N-oxidation-2,6-dichloropyridine obtains compound (6) by reflux reaction in pyridine; Then in three Reflux reduction in phosphorus chloride and chloroform solvent obtains 7-(6-chloropyridyl)-7-oxygen, sulfur and azacoumarin compound (7); simultaneously compound (5) is passed through potassium carbonate and acetone room temperature condition React with various alkyl, substituted aryl and substituted heterocyclic halides to obtain differently substituted 7-oxo, sulfur and aza coumarin compounds (8),
[0030] General formula one:
[0031]
[0032] 2. According to general formula two, synthetic starting material (9) (referring to reference (European Journal of Medicinal Ch...
Embodiment 2
[0036] According to the general formula 1, a compound 13 in compound type 6 was synthesized
[0037]
[0038]7-mercapto-4-methyl-chromen-2-one (5,300mg, 1.56mmol) and 2,6-dichloropyridine N-oxide (256mg, 1.56mmol) were refluxed in pyridine (6mL) solvent for 2 hour; Then add 2 equivalents of hydrochloric acid (50mL), filter and distill off the solvent under reduced pressure, separate with chromatographic column (eluent is chloroform / ethyl acetate=99 / 1) to obtain 240mg product 13, yield 48%, melting point It is 236-237°C. 1 H NMR (CDCl 3 ):δ2.50(3H,s,4-CH 3 ),6.41(1H,s,3-H),6.55(1H,dd,J1=8.1Hz,J2=1.8Hz,6-H),7.01(1H,t,J=8.4Hz,H of pyridine), 7.28, 7.54 (each 1H, dd, J1 = 8.4Hz, J2 = 1.8Hz, 2H of pyridine), 7.62 (1H, d, J = 1.8Hz, 8-H), 7.73 (1H, d, J = 8.1Hz ,5-H). 13 C NMRδ18.83,58.42,116.76,120.27,122.30,124.00,125.77,126.45,131.11,133.28,141.80,151.75,154.05,154.91,159.97. ESI MS m / z 320(M + +1).
Embodiment 3
[0040] According to general formula 1, a compound 4 in compound type 7 is synthesized,
[0041]
[0042] Compound 13 (500mg, 1.56mmol) was dissolved in phosphorus trichloride (PCl 3 , 2mL) and chloroform (CHCl 3 , 20mL), after reflux for 1.5 hours, add ice water (200mL) to dilute the reaction solution, extract with dichloromethane (30mL×4), combine the organic phases and dry with anhydrous sodium sulfate, filter, remove the solvent under reduced pressure and then chromatograph Column separation (eluent is chloroform / methanol=20 / 1) to obtain 237mg product 4, yield 50%, melting point is 138-140 ℃. 1 H NMR (CDCl 3 )δ2.46(3H,s,4-CH 3 ),6.33(1H,s,3-H),7.01,7.13,7.52(each 1H,d,J=7.8Hz,3H of pyridine),7.45(1H,d,J=8.4Hz,6-H), 7.48(1H,s,8-H),7.62(1H,d,J=8.4Hz,5-H). 13 C NMRδ18.94,116.55,121.00,122.05,122.26,122.31,126.24,129.81,136.78,140.25,152.41,152.80,154.75,160.35,161.25.ESI MS m / z 304(M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com