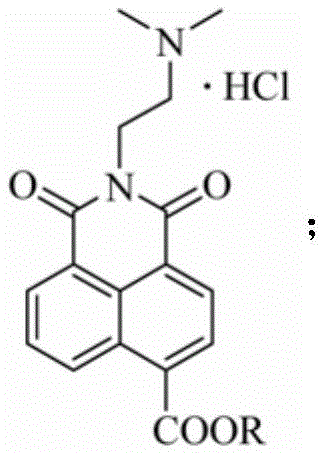
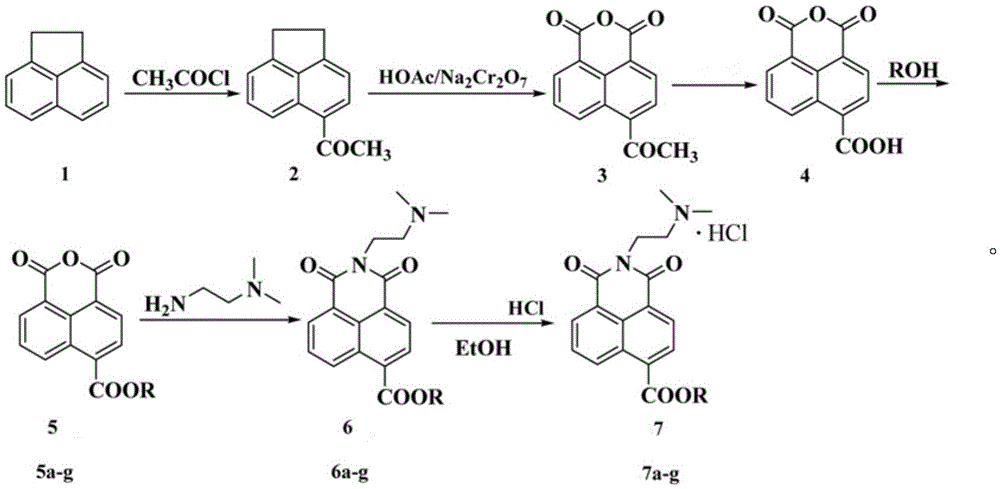
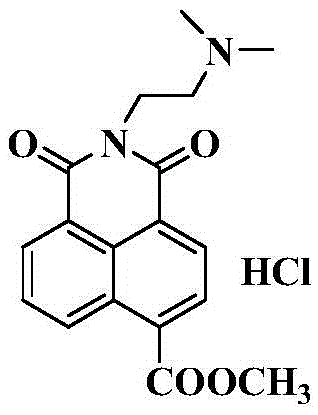
Naphthalimide derivative using ester group for modifying amonafide naphthalene ring and preparation method and application of naphthalimide derivative
A technology of aminonaphthyl naphthalene ring and naphthalene imide, which is applied in the field of naphthalene imide derivatives and its preparation, to achieve good inhibitory ability and avoid unpredictable toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 4-Formylmethyl-2-[2-(dimethylamino)-ethyl]1H-benzisoquinoline-1,3(2H)-dione hydrochloride (compound 7a)
[0023]
[0024] The preparation method is as follows:
[0025] (1) 3.6g (27mmol) AlCl 3 Dissolve in 20mL of dried dichloromethane, add 2.78g (27mmol) of acenaphthene (compound 1), control the temperature below 15°C, slowly add 1.4g (27mmol) of acetyl chloride in 10mL of dichloromethane solution, and the addition is complete. Continue the reaction for 1 h, add ice water to the reaction bottle to terminate the reaction, extract with dichloromethane, dry and concentrate, petroleum ether: ethyl acetate = 4:1 silica gel column separation and purification to obtain compound 2:
[0026] (2) Dissolve 1.96g (10mmol) of compound 2 in 22mL of glacial acetic acid, add 8.01g (27mmol) of sodium dichromate, control the reaction temperature at 90-100°C for 2h, then cool to room temperature, pour the reaction solution into ice water, Acidify with hydrochloric acid to pH 2-3, fi...
Embodiment 2
[0033] 4-Formyl ethyl ester-2-[2-(dimethylamino)-ethyl]1H-benzisoquinoline-1,3(2H)-dione hydrochloride (7b)
[0034]
[0035] Except that methanol is replaced with ethanol in the (4) step, other synthesis and purification methods are the same as in Example 1.
[0036] Yield: 59%, 1 HNMR (400MHz,D 2 O) δ8.56(d, J=53.0Hz, 1H), 8.03(d, J=11.43Hz, 3H), 7.58(d, J=36.1Hz, 1H), 4.46(d, J=7.0Hz, 2H ), 4.36(d, J=9.5Hz, 2H), 3.50~3.37(m, 2H), 3.16~2.79(m, 6H), 1.44(t, J=8.9Hz, 3H).ESI-MSm / z: 341.1 [M+1–HCl] + .Anal.calcdforC 19 h 21 ClN 2 o 4 1.1H 2 O: C57.53%, H5.90%, N7.06%; found C57.66%, H5.81%, N7.03%.
Embodiment 3
[0038] 4-Formylpropyl-2-[2-(dimethylamino)-ethyl]1H-benzisoquinoline-1,3(2H)-dione hydrochloride (7c)
[0039]
[0040] Except that methanol is replaced with propanol in the (4) step, other synthesis and purification methods are the same as in Example 1.
[0041] Yield: 42%, 1 HNMR (400MHz,D 2 O)δ8.52(s,1H),8.35~7.70(m,3H),7.53(s,1H),4.30(s,4H),3.36(d,J=6.1Hz,2H),3.17~2.60( m,6H),1.95~1.60(m,2H),1.17~0.71(m,3H).ESI-MSm / z:355.1[M+1–HCl] + .Anal.calcdforC 20 h 23 ClN 2 o 4 0.7H 2 O: C59.54%, H6.10%, N6.94%; found C59.38%, H6.31%, N6.89%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap