Naphthalimide derivative and preparing method and application thereof
A technology of naphthalimide and derivatives, applied in the field of naphthalimide derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
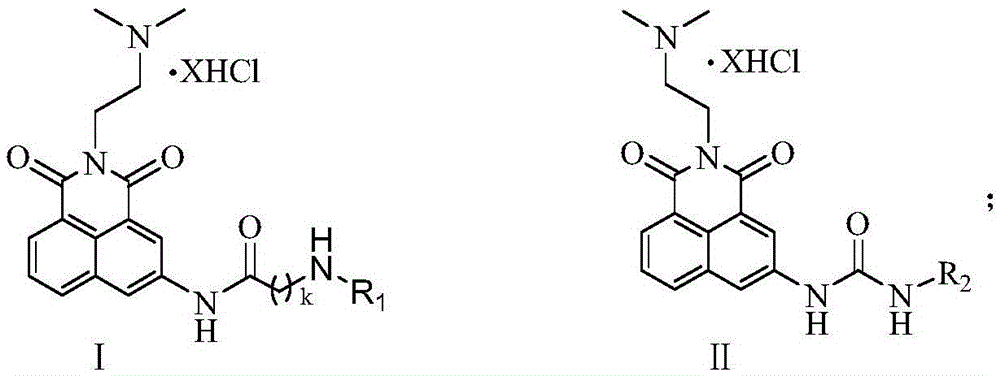
[0021] 2-{3-[3-(3-Aminopropyl)-aminopropyl]-aminopropyl}-2-[(2-dimethylamino)-ethyl]1H-benzisoquinoline-1 Preparation of 3(2H)-diketoacetamide pentahydrochloride (17a):
[0022]
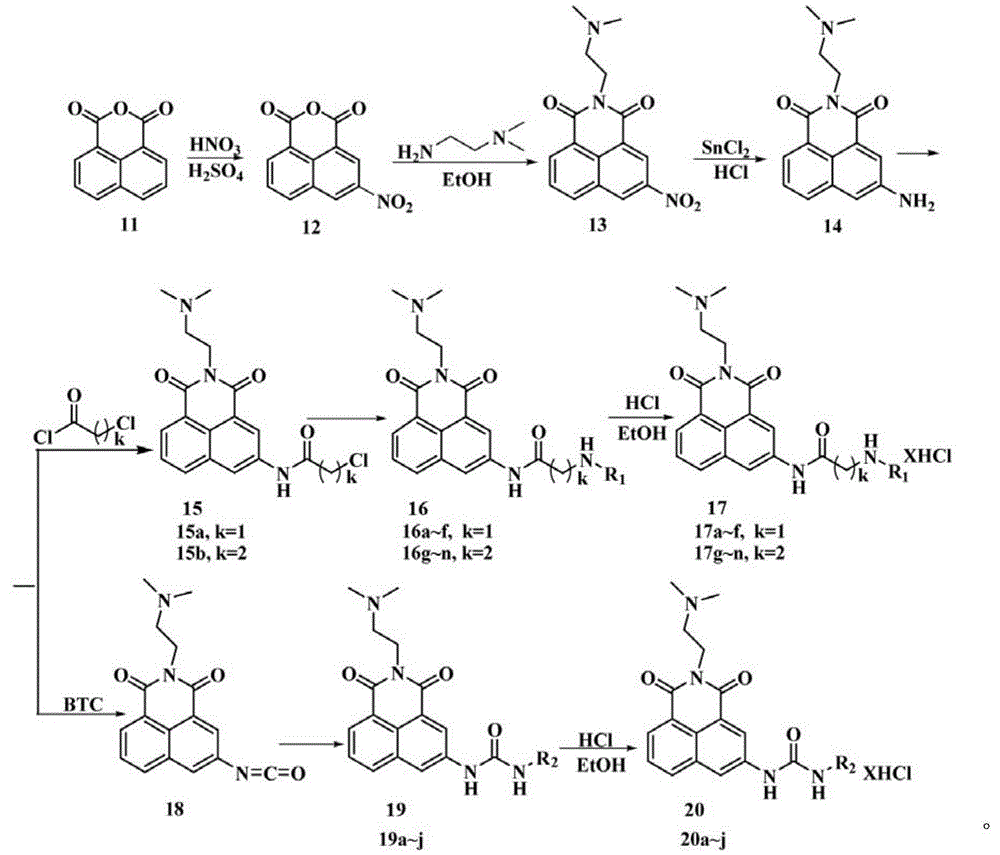
[0023] (1) Take 5mmol (1.04g) of compound 14, 7.5mmol (1.0g) of anhydrous potassium carbonate in 50ml of acetonitrile, slowly add 5ml of acetonitrile solution of chloroacetyl chloride (containing 6mmol of chloroacetyl chloride) with stirring at room temperature. , Stirring at room temperature overnight, the reaction is over, suction filtration under reduced pressure, collecting the solid, washing with acetonitrile, and drying to obtain compound 15a;
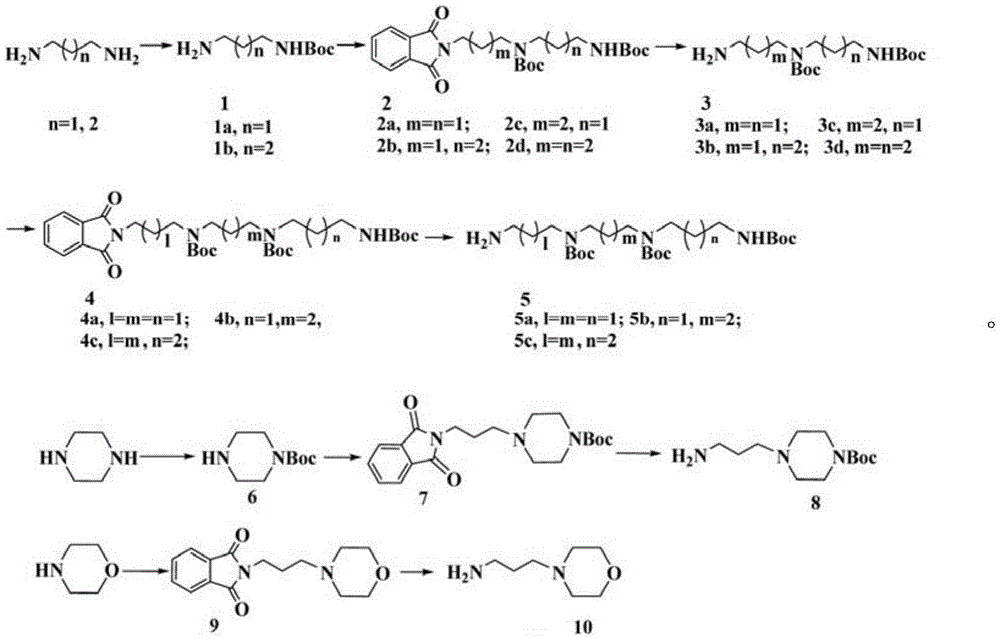
[0024] (2) 1mmol of compound 15a and 1.5mmol (0.2g) of anhydrous potassium carbonate in 15ml of dry acetonitrile, add 5ml of N,N-dimethylformamide to the mixed solution, stir at 45℃ for 15min, add 5a1mmol, 45℃ Stir under conditions and monitor by TLC. After the reaction, the solvent was evaporated under reduced pressure, extracted with chloroform, washed wi...
Embodiment 2
[0027] 2-{3-[4-(3-Aminopropyl)-aminobutyl]-aminopropyl}-2-[(2-dimethylamino)-ethyl]1H-benzisoquinoline-1 The preparation of 3(2H)-diketoacetamide pentahydrochloride (17b):
[0028]
[0029] Except that 5b is used instead of 5a in step (2), other synthesis and purification methods are the same as in Example 1. Yield: 78%, white solid: 1 HNMR(400MHz, D 2 O)δ: 8.21(d,J=7.2Hz,1H,Ar-H); 8.18(s,2H,Ar-H); 8.07(d,J=7.6Hz,1H,Ar-H); 7.65(t ,J=7.8Hz,1H,Ar-H); 4.38(t,J=6.2Hz,2H,1×CH 2 ); 4.20(s,2H,1×CH 2 ); 3.44(t,J=6.2Hz,2H,1×CH 2 ); 3.30(t,J=8.0Hz,2H,1×CH 2 ); 3.18(t,J=8.0Hz,2H,1×CH 2 ); 3.01~3.12(m,8H,4×CH 2 ); 2.96(s,6H,2×CH 3 ); 2.18~2.22(m,2H,1×CH 2 ); 2.01~2.05(m,2H,1×CH 2 ); 1.73~1.75(m,2H,1×CH 2 ).ESI-MIm / z:526.4[M+1-5HCl] + .Anal.calcd.forC 28 H 48 Cl 5 N 7 O 3 : C47.50%, H6.83%, N13.85%; found: C47.4%, H6.78%, N13.98%.
Embodiment 3
[0031] 2-{4-[4-(4-Aminobutyl)-aminobutyl]-aminobutyl}-2-[2-(dimethylamino)-ethyl]1H-benzisoquinoline-1 Preparation of 3(2H)-diketoacetamide pentahydrochloride (17c):
[0032]
[0033] Except that 5d is used instead of 5a in step (2), the other synthesis and purification methods are the same as in Example 1. Yield: 75%, white solid: 1 HNMR(400MHz, D 2 O)δ: 8.09(d,J=7.2Hz,1H,Ar-H); 8.0(d,J=2.0,2H,Ar-H); 7.97(d,J=2.0Hz,1H,Ar-H) ;7.94(d,J=8.0Hz,1H,Ar-H); 7.58(t,J=7.8,1H,Ar-H); 4.32(t,J=6.0Hz,2H,1×CH 2 ); 4.18(s,2H,1×CH 2 ); 3.43(t,J=6.4Hz,2H,1×CH 2 ); 3.26(t,J=7.6Hz,2H,1×CH 2 ); 3.03~3.13(m,8H,4×CH 2 ); 2.98(s,6H,3×CH 3 ); 1.79~1.93(m,4H,2×CH 2 ); 1.70~1.76(m,4H,2×CH 2 ).ESI-MIm / z:554.4[M+1-5HCl] + .Anal.calcd.forC 30 H 52 Cl 5 N 7 O 3 : C48.95%, H7.12%, N13.32%; found: C48.89%, H7.23%, N13.28%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com