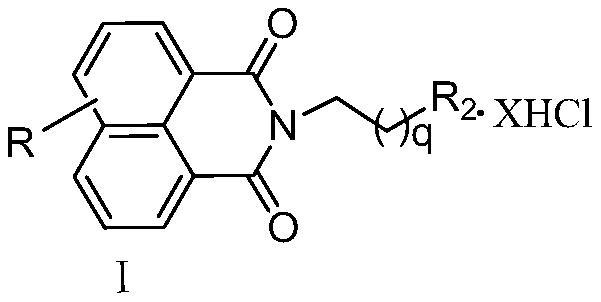
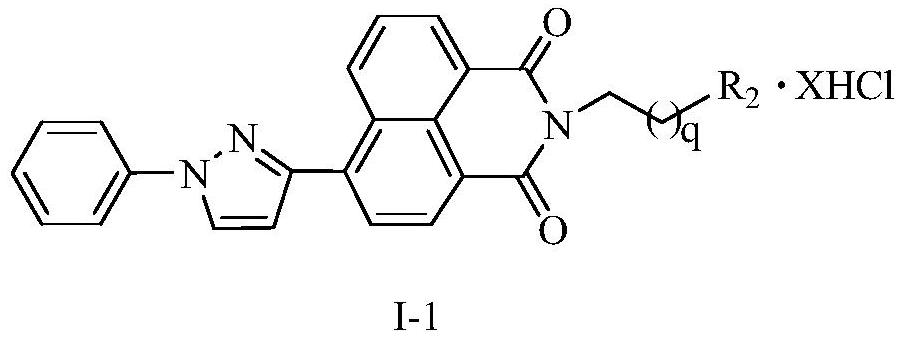
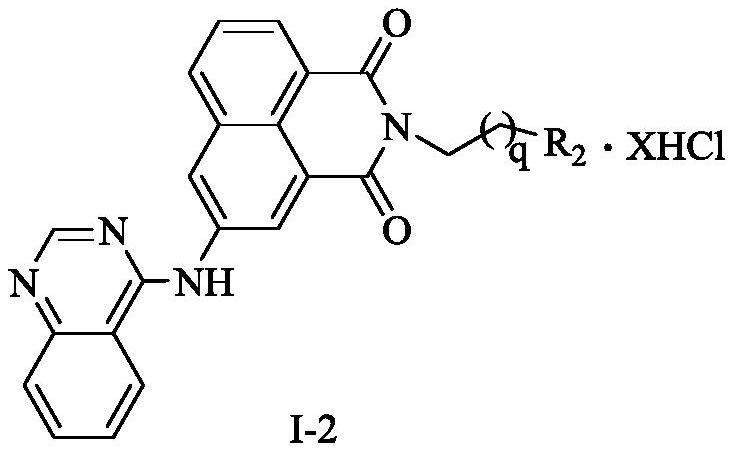
A kind of naphthalimide-polyamine conjugate and its preparation method and application
A naphthalimide and conjugate technology, which is applied in the field of naphthalimide-polyamine conjugates and their preparation, can solve the problems of difficulty in reducing or eliminating the toxic and side effects of myelosuppression, and achieves good development potential and novel skeleton. , good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 6{3-(1-phenyl-1H-pyrazole)}-2-[4-4-(4-aminobutyl)-aminobutyl-aminobutyl]1H-benzisoquinoline-1, Synthesis of 3(2H)-diketone tetrahydrochloride (14a)
[0046]
[0047] (1) Take 1.0g (6.5mmol) compound 8, anhydrous AlCl 3 1.29g (9.7mmol) was placed in a 100mL round-bottomed flask, and dry dichloromethane was added as a reaction solvent. After stirring at room temperature for 15 minutes, 0.48mL of a dichloromethane solution containing 6.8mmol of acetyl chloride was slowly added dropwise under ice-bath conditions. After that, react at room temperature for 30 minutes. After 2 hours, it was poured into ice water, the organic layer was extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated under reduced pressure, petroleum ether: ethyl acetate = 10:1 column separation and purification to obtain compound 9;
[0048] (2) Weigh 1.3g (6.7mmol) of compound 9 obtained in step (1) into a 50mL round bottom flask, add 7mL DMF-DMA as a reactant and as a s...
Embodiment 2
[0054] 6{3-(1-phenyl-1H-pyrazole)}-2-[3-3-(3-aminopropyl)-aminopropyl-aminopropyl]1H-benzisoquinoline-1, Synthesis of 3(2H)-diketone tetrahydrochloride (14b)
[0055]
[0056] Except replacing 5b with 5a in step (4), other synthesis and purification methods are the same as in Example 1. Yield 52%.
[0057] 1 H NMR (300MHz, DMSO-d6) δ: 8.46 (dd, J=9.0, 6.0Hz, 2H), 8.08 (d, J=9.0Hz, 1H), 7.98 (d, J=3.0Hz, 1H), 7.77 (dd,J=15.0,7.5Hz,2H),7.25-7.18(m,5H),6.83(s,1H),4.10(t,J=6.0Hz,2H),3.01-2.88(m,10H), 2.08-1.97(m,6H).13C NMR(75MHz,DMSO-d6)δ163.96,163.75,141.04,139.72,139.17,134.96,132.06,131.49,130.37,130.17,130.01,129.56,118.415,12 123.04,122.93,111.44,45.25,44.30,44.22,37.71,36.32,24.88,23.89,22.63.ESI-MS m / z:511.47[M-4HCl+1] + .Elemental analysis for C 30 h 38 C l4 N 6 o 2 : C, 54.89; H, 5.83; N, 12.80; Found: C, 54.73; H, 6.18; N, 12.73.
Embodiment 3
[0059] 6{3-(1-phenyl-1H-pyrazole)}-2-[4-(4-aminobutyl)-aminobutyl]1H-benzisoquinoline-1,3(2H)-two Synthesis of Ketone Trihydrochloride (14c)
[0060]
[0061] Except that 3d is used to replace 5b in step (4), other synthesis and purification methods are the same as in Example 1. Yield 58%.
[0062] 1 H NMR (300MHz, DMSO-d6) δ: 8.49-8.41 (m, 2H), 8.12-8.03 (m, 1H), 7.98 (d, J = 3.0Hz, 1H), 7.77 (dd, J = 13.5, 7.5 Hz, 2H), 7.25-7.17(m, 5H), 6.84(d, J=3.0Hz, 1H), 4.05(s, 2H), 2.95-2.82(m, 4H), 2.79(d, J=6.0Hz ,2H),1.66(d,J=18.0Hz,8H). 13 C NMR(75MHz,DMSO-d6)δ163.77,163.58,141.05,139.70,139.16,134.96,132.08,131.53,130.42,130.20,130.00,129.56,128.48,128.06,124.73,122.94,122.83,111.43,46.74,46.26,38.28 ,25.21,24.34,23.59,22.84.ESI-MS m / z:482.43[M-3HCl+1] + .Elemental analysis for C 29 h 34 Cl 3 N 5 o 2 0.3H 2 O: C, 58.41; H, 5.85; N, 11.74; Found: C, 58.92; H, 5.52; N, 11.58.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com