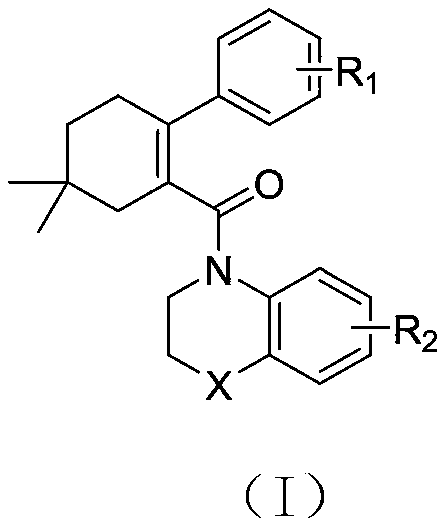
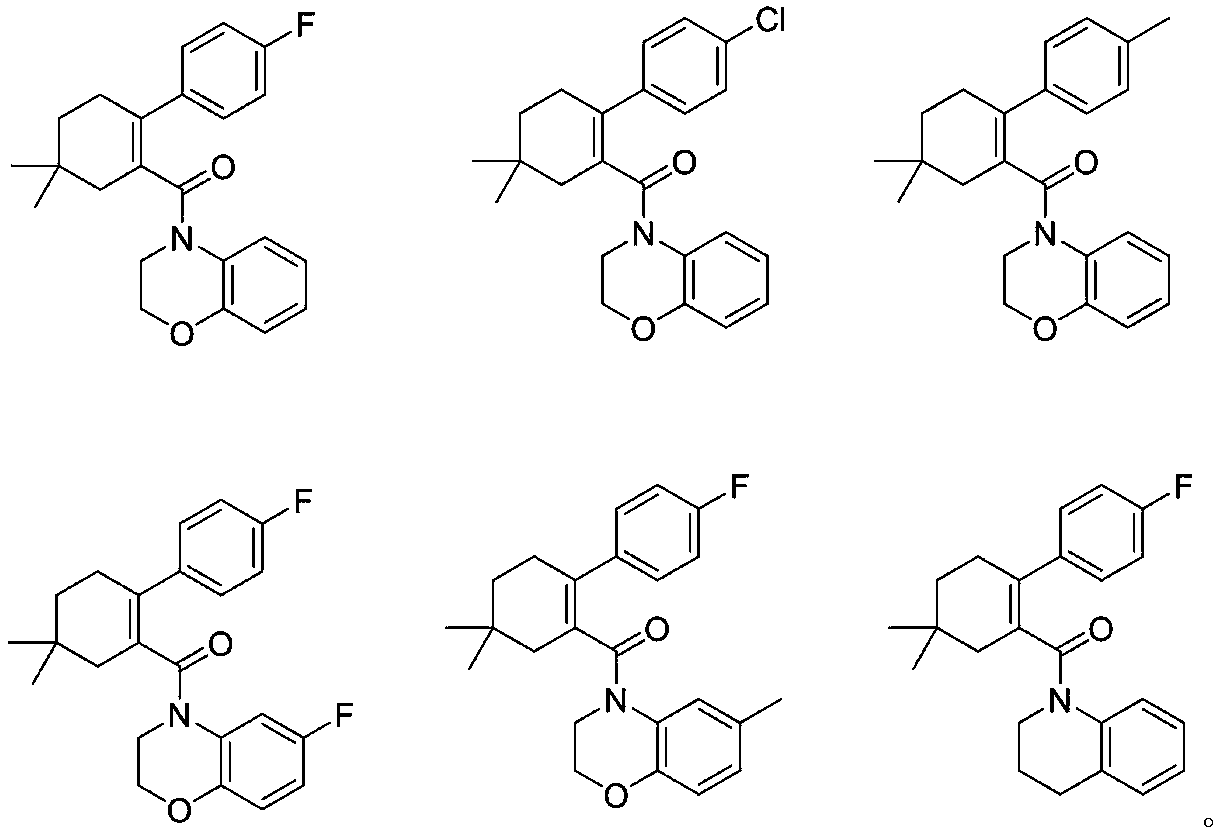
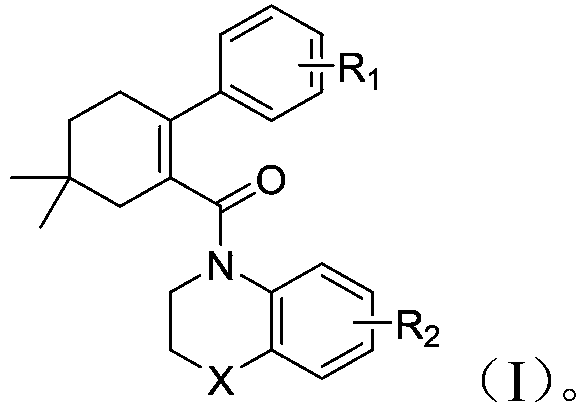
5,5-dimethylcyclohexyl-1-ene derivative and application thereof in metabolic diseases
A technology of dimethylcyclohexane and derivatives, applied in the field of medicine, to achieve the effect of great research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] (1) Preparation of 2-bromo-5,5-dimethylcyclohex-1-enylcarbaldehyde
[0027] Add 2.1mL (30.0mmol) of N,N-dimethylformamide into 10mL of chloroform, after cooling down to 0°C, slowly add 2.8mL (27.0mmol) of phosphorus tribromide, continue stirring at 0°C for 2h, then batch 1.3 g (10.0 mmol) of 4,4-dimethylcyclohexanone was added, and the mixture was raised to room temperature for 6 h. TLC detected that the reaction was complete, and the reaction solution was slowly poured into ice water, and the reaction solution was adjusted to pH=7 with sodium bicarbonate. Extract with dichloromethane, combine the organic phases, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate. The desiccant was filtered off, concentrated under reduced pressure, and the residue was purified by silica gel column chromatography to obtain 1.2 g of light yellow oil with a yield of 54.8%.
[0028] (2) Preparation of 2-(4-fluorophenyl)-5,5-dimethylcyclohex-1-en...
Embodiment 2
[0036]
[0037] 1 H-NMR (400MHz, DMSO-d 6 )δ7.44-7.38(m,2H),7.28(d,J=7.5Hz,2H),7.13-7.04(m,3H),4.36(t,J=7.1Hz,2H),4.10(t,J =7.1Hz, 2H), 2.57–2.52(m, 1H), 2.30-2.28(m, 1H), 1.96~1.92(m, 2H), 1.36(t, J=6.4Hz, 2H), 0.93(s, 6H).MS(ESI)m / z 382.4[M+H] + .
Embodiment 3
[0039]
[0040] 1 H-NMR (400MHz, DMSO-d 6 )δ7.38(d,J=7.4Hz,1H),7.20–7.15(m,3H),7.04-6.96(m,4H),4.36(t,J=7.2Hz,2H),4.07(t,J =7.1Hz, 2H), 2.55–2.52(m, 1H), 2.37(s, 3H), 2.29-2.25(m, 1H), 1.96~1.91(m, 2H), 1.36(t, J=6.4Hz, 2H),0.94(s,6H).MS(ESI)m / z362.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com