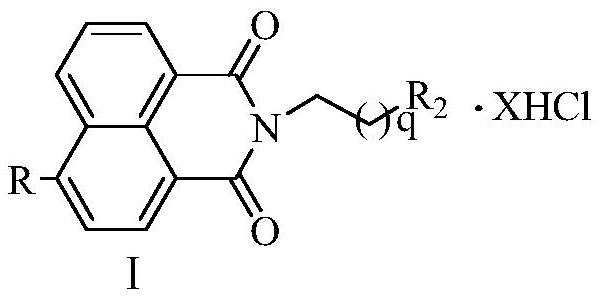
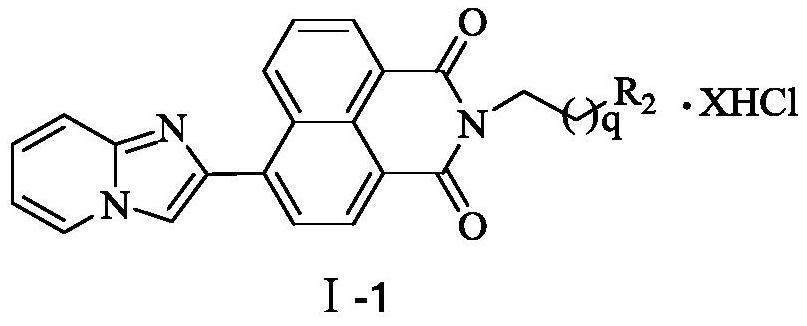
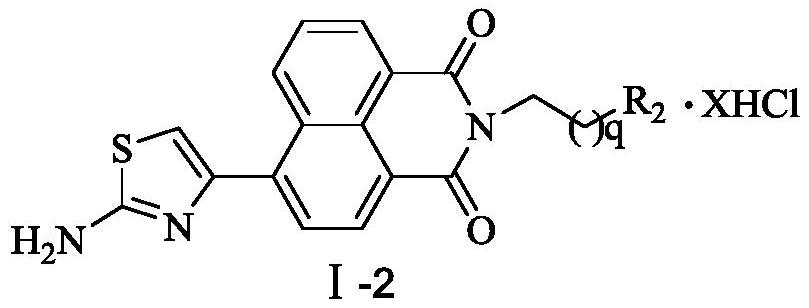
A kind of aromatic heterocyclic modified naphthalimide derivative and preparation method and use thereof
A naphthalene imide and aromatic heterocycle technology, applied in the field of medicinal chemistry, can solve the problems such as dose toxicity and side effects failed to be successfully marketed, and achieve the effects of good development potential, good inhibitory activity, and novel skeleton
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 6-[1-(imidazo[1,2-a]pyridine)]-2-{4-[4-(4-aminobutyl)-aminobutyl]-aminobutyl}-1H-benzoiso Synthesis of quinoline-1,3(2H)-dione tetrahydrochloride (16a)
[0046]
[0047] (1) Take 1.0g (6.5mmol) compound 10 and anhydrous AlCl 3 1.29g (9.7mmol) was placed in a 100mL round-bottomed flask, and dry dichloromethane was added as a reaction solvent. After stirring at room temperature for 15 minutes, a dichloromethane solution of 0.48mL (6.8mmol) of acetyl chloride was slowly added dropwise under ice-bath conditions. After dropping, react at room temperature for 30 minutes. After 2 hours, it was poured into ice water, the organic layer was extracted with dichloromethane, dried over anhydrous sodium sulfate, concentrated under reduced pressure, petroleum ether: ethyl acetate = 10:1 column separation and purification to obtain compound 11;
[0048] (2) Take 2.2g (7.5mmol) of compound 11 obtained in step (1), K 2 Cr 2 o 7 2H 2 O 6.6g (22.0mmol) was refluxed in 20mL glacia...
Embodiment 2
[0055] 6-[1-(imidazo[1,2-a]pyridine)]-2-[4-(4-aminobutyl)-aminobutyl]-1H-benzisoquinoline-1,3(2H )-Synthesis of diketone trihydrochloride (16b)
[0056]
[0057] Except replacing 5a with 3b in step (5), other synthesis and purification methods are the same as in Example 1. Yield 52%.
[0058] 1 H NMR (300MHz, Deuterium Oxide) δ: 8.60 (d, J = 6.0Hz, 1H), 8.31 (s, 1H), 8.24 (dd, J = 12.0, 6.0Hz, 3H), 7.96–7.84 (m, 1H ),7.75(t,J=7.5Hz,2H),7.63(t,J=7.5Hz,1H),7.42(s,1H),3.95(t,J=6.0Hz,2H),3.25–3.05(m ,6H),2.06-2.16(m,2H),1.86–1.63(m,4H). 13 C NMR(75MHz,Deuterium Oxide)δ164.55,164.19,140.54,134.10,132.10,131.79,131.22,130.60,130.46,128.50,128.37,127.66,127.08,121.95,121.09,117.46,114.72,112.01,47.31,44.47,39.78, 36.52, 24.17, 23.73, 23.16. ESI-MS m / z: 442.45[M-3HCl+1] + .Elemental analysis for C 26 h 30 Cl 3 N 5 o 2 2.9H 2 O: C, 51.78; H, 5.98; N, 11.61; Found: C, 51.89; H, 6.09; N, 11.45.
Embodiment 3
[0060] 6-[1-(imidazo[1,2-a]pyridine)]-2-[3-(3-aminopropyl)-aminopropyl]-1H-benzisoquinoline-1,3(2H )-Diketone Trihydrochloride (16c) Synthesis
[0061]
[0062] Except replacing 5a with 3a in step (5), other synthesis and purification methods are the same as in Example 1. Yield 59%.
[0063] 1 H NMR (300MHz, Deuterium Oxide) δ8.70(d, J=6.0Hz, 1H), 8.43(s, 1H), 8.35-8.24(m, 3H), 8.03-7.94(m, 1H), 7.84(dd ,J=9.0,3.0Hz,2H),7.68(t,J=9.0Hz,1H),7.50(t,J=6.0Hz,1H),4.07(t,J=7.5Hz,2H),3.22-3.13 (m,6H),2.29-1.90(m,4H). 13 C NMR(75MHz,Deuterium Oxide)δ164.60,164.25,140.43,134.56,131.92,131.67,131.38,130.57,130.50,128.78,128.70,128.48,127.86,127.17,122.04,121.04,117.73,114.88,111.88,45.44,44.63, 37.46,36.51,24.22,23.73.ESI-MS m / z:428.41[M-3HCl+1] + .Elemental analysis for C 25 h 28 Cl 3 N 5 o 2 3.2H 2 O: C, 50.51; H, 5.83; N, 11.78; Found: C, 50.56; H, 5.92; N, 11.62.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com