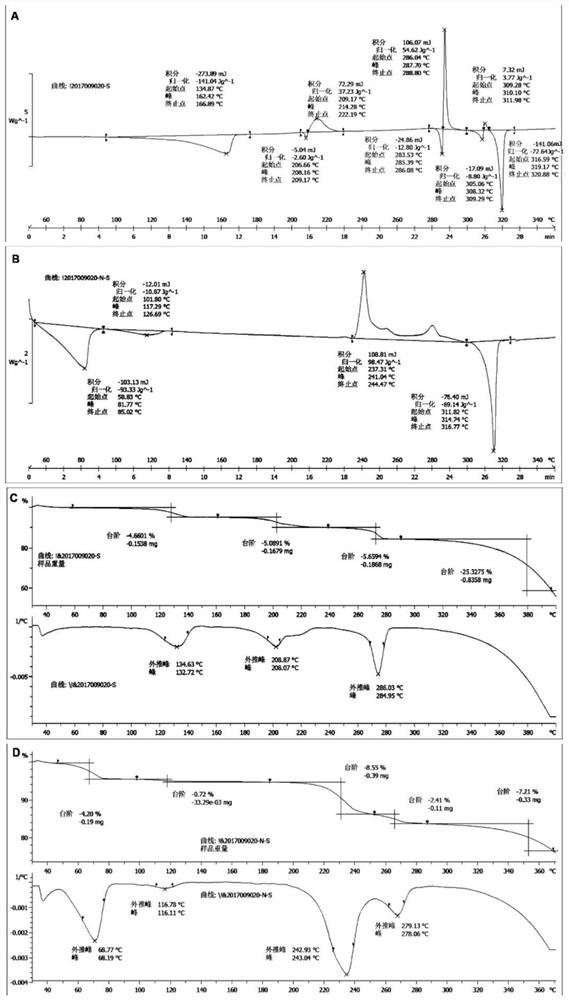
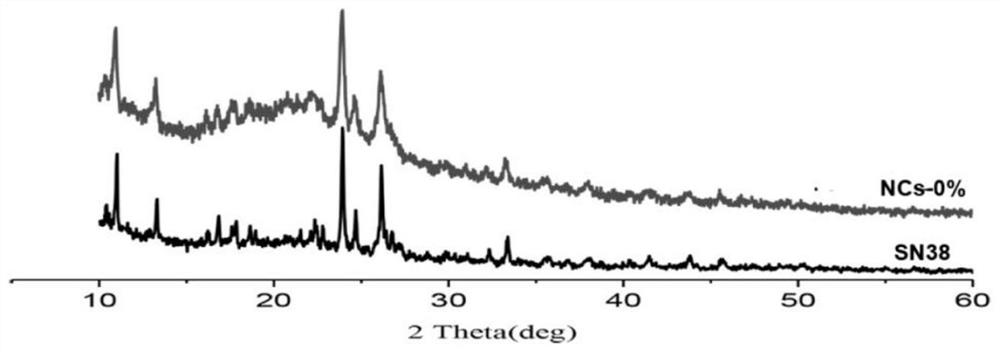
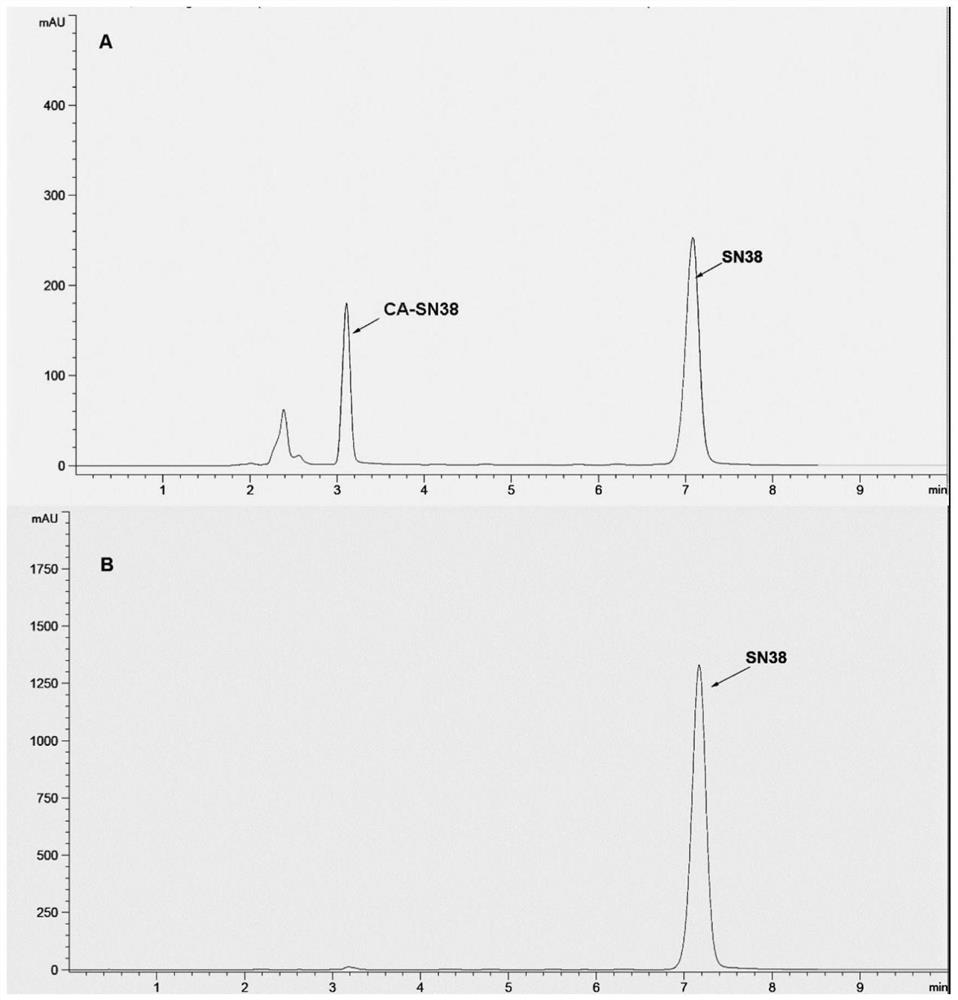
Preparation of Camptothecin Drug Nanocrystals by Reversible Decomposition
A technology of nanocrystals and camptothecin, applied in the field of medicine, can solve the problems of limiting the clinical application of nanocrystal preparations, rapid growth of particle size, etc., and achieve the effects of retaining antitumor activity, high drug loading, and low requirements for preparation instruments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Preparation of SN38 prototype / carboxylic acid type mixture
[0046] Take 1 mL of ammonia water, dissolve it in 5 mL of water, weigh SN38 (0.2 g) and disperse it in the aqueous ammonia water solution, and react in the dark at room temperature until the solution is clear, stop the reaction, remove the reaction solvent by rotary evaporation under reduced pressure at 60°C to obtain SN38 prototype / carboxylic acid Type mixture (light yellow flaky solid), wherein the mass percentage of prototype drug is 82.1%.
[0047] (2) Preparation of SN38 nanocrystal preparation
[0048] The mixture (20 mg) obtained in step (1) was dispersed in 20 mL of purified water, and the probe was sonicated for 10 min (100 W) to obtain a blue-green opalescent solution. Subsequently, the preparation solution is filtered through a 0.22 μm filter membrane and freeze-dried to obtain a freeze-dried powder of SN38 nanocrystals. Take 20 mL of 5% glucose solution for injection to redissolve the freeze-...
Embodiment 2
[0050] (1) Preparation of camptothecin drug prototype / carboxylic acid type mixture
[0051] Take 2 mL of ammonia water, dissolve it in 5 mL of water, weigh camptothecin drugs (0.2 g, including CPT-11, HCPT, CDK-602 or TPT) and disperse them in the aqueous ammonia water solution, and react at room temperature in the dark until the solution is clear and stop the reaction , After removing the reaction solvent by rotary evaporation under reduced pressure at 60°C, solid powder or suspension (light yellow flaky solid or light yellow solution) of camptothecin drug prototype / carboxylic acid mixture was obtained.
[0052] (2) Preparation of camptothecin drug nanocrystal preparation
[0053] Disperse the mixture (20 mg) obtained in step (1) in 20 mL of purified water (or dilute the suspension to 250 mL, measure 25 mL), and sonicate the probe for 10 min (100 W) to obtain a blue-green opalescent solution. Subsequently, the preparation solution is filtered through a 0.22 μm filter membran...
Embodiment 3
[0057] (1) Preparation of SN38 prototype / carboxylic acid type mixture
[0058] Take 4 mL of triethylamine, dissolve it in 4 mL of water, weigh SN38 (0.2 g) and disperse it in the aqueous solution of triethylamine, stir at room temperature in the dark until the solution is clear, stop the reaction, and dry in vacuum to remove most of the alkaline reagents to obtain SN38 Prototype / carboxylic acid type mixture suspension (light yellow liquid), wherein the mass percentage of the prototype drug is 18.3%.
[0059] (2) Preparation of SN38 nanocrystal preparation
[0060] Set the volume of the mixture suspension prepared in step (1) to 50 mL, take (25 mL of aqueous solution (50 mg of human serum albumin was added), and stir rapidly to obtain a blue-green opalescent preparation solution. Then the preparation solution was freeze-dried to obtain SN38 / albumin nanocrystal freeze-dried powder. Take 30mL of 0.9% normal saline to redissolve the freeze-dried powder, shake and disperse to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com