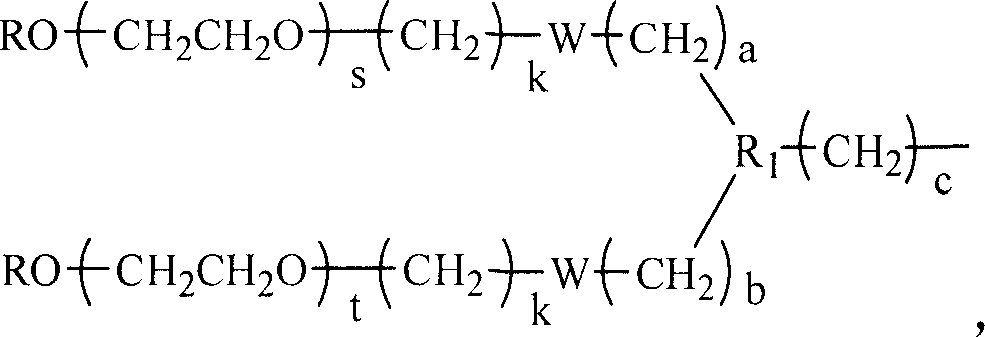High molecular antineoplastic cisplatin medicine and preparation method thereof
An anti-tumor drug, polymer technology, applied in the field of medicine, can solve problems such as large toxic side effects and fast metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] RPEG 2 The synthesis method of CO-B-C (take monomethoxypolyethylene glycol (mPEG) molecular weight 2000 as an example) 1. Dissolve 10g mPEG (Mn=2000, 5mmol) in about 150mL toluene, and distill it under atmospheric pressure under the protection of nitrogen most of the toluene to remove trace moisture contained in mPEG. After cooling to room temperature, about 200 mL of anhydrous THF was added thereto, heated to a slight boil until mPEG was completely dissolved. Subsequently, 0.2 mol / L sodium naphthalene solution was added dropwise to the mPEG solution until the solution was gray-green. 5.24 mL of ethyl chloroacetate (6.03 g, 50 mmol) was diluted with 50 mL of THF and added to the reaction solution, and stirred at room temperature for 4 h. After the reaction solution was concentrated, it was precipitated with anhydrous ether, and the obtained crude product was added to 300 mL of 0.1 mol / L NaOH solution, and stirred at 50°C for 12 h. Then use 1mol / L HCl to adjust pH=3, ...
Embodiment 2
[0057] Preparation of RPEGCO-B-C-Pt (taking mPEG molecular weight 4000 as an example)
[0058] Weigh 10g of mPEG (Mn=4000, 2.5mmol), prepare the mPEG-CH with carboxyl group in the same way as in Example 1 2 COOH, linked with diethyl aminomalonate, hydrolyzed, and finally synthesized mPEG-CH 2 CO-Lysine-Pt. The yield was 81%, and the molar content of cisplatin in the final product was 85.2%.
Embodiment 3
[0060] RPEG 2 The synthesis method of CO-B-C (taking monomethoxypolyethylene glycol (mPEG) molecular weight 80000 as an example) 16g mPEG (Mn=80000, 0.2mmol) was obtained in the same way as in Example 1 to obtain mPEG-CH 2 CO-Lysine-Pt 4.5g, total yield 28%. The molar content of cisplatin in the final product was 80.4%.
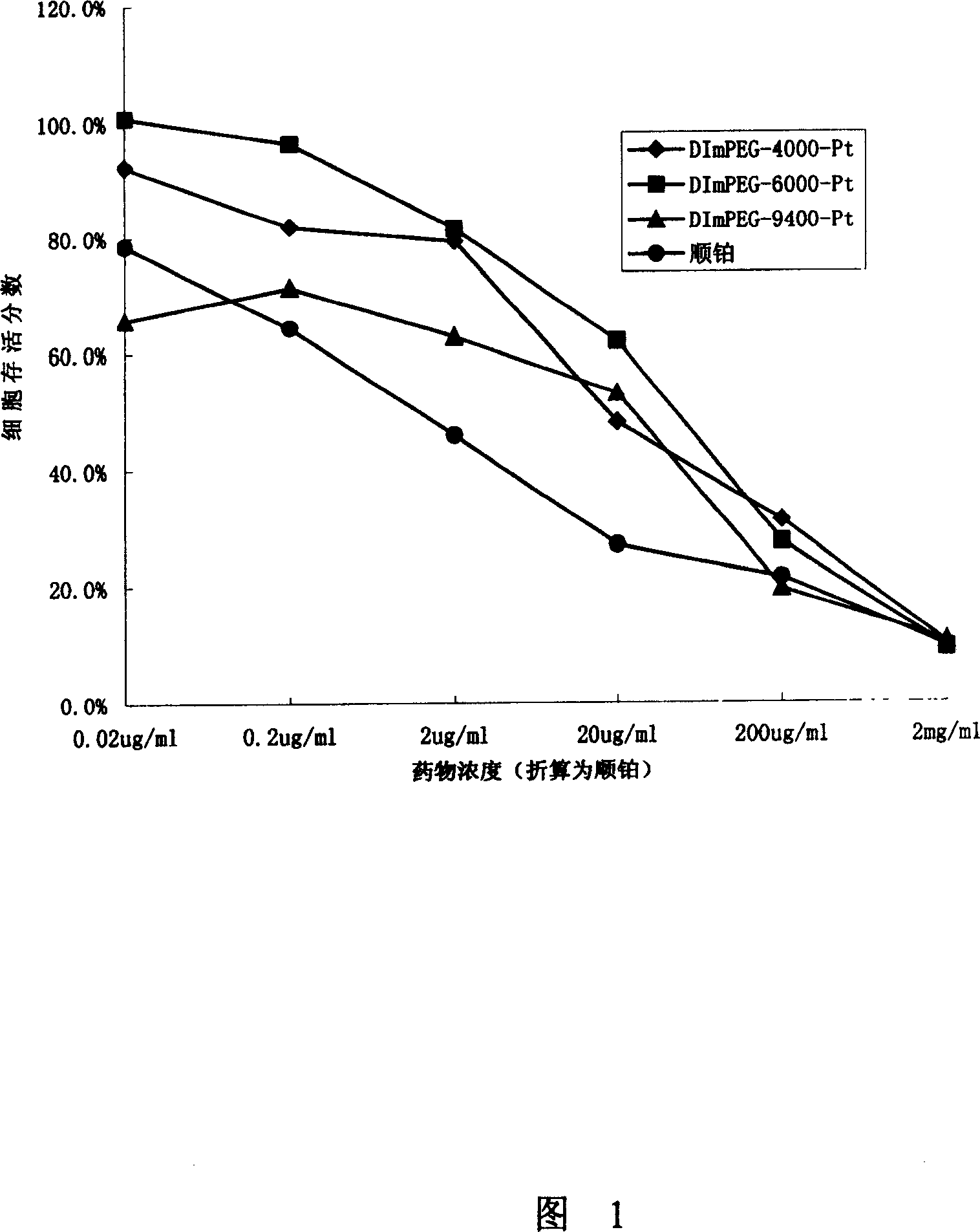
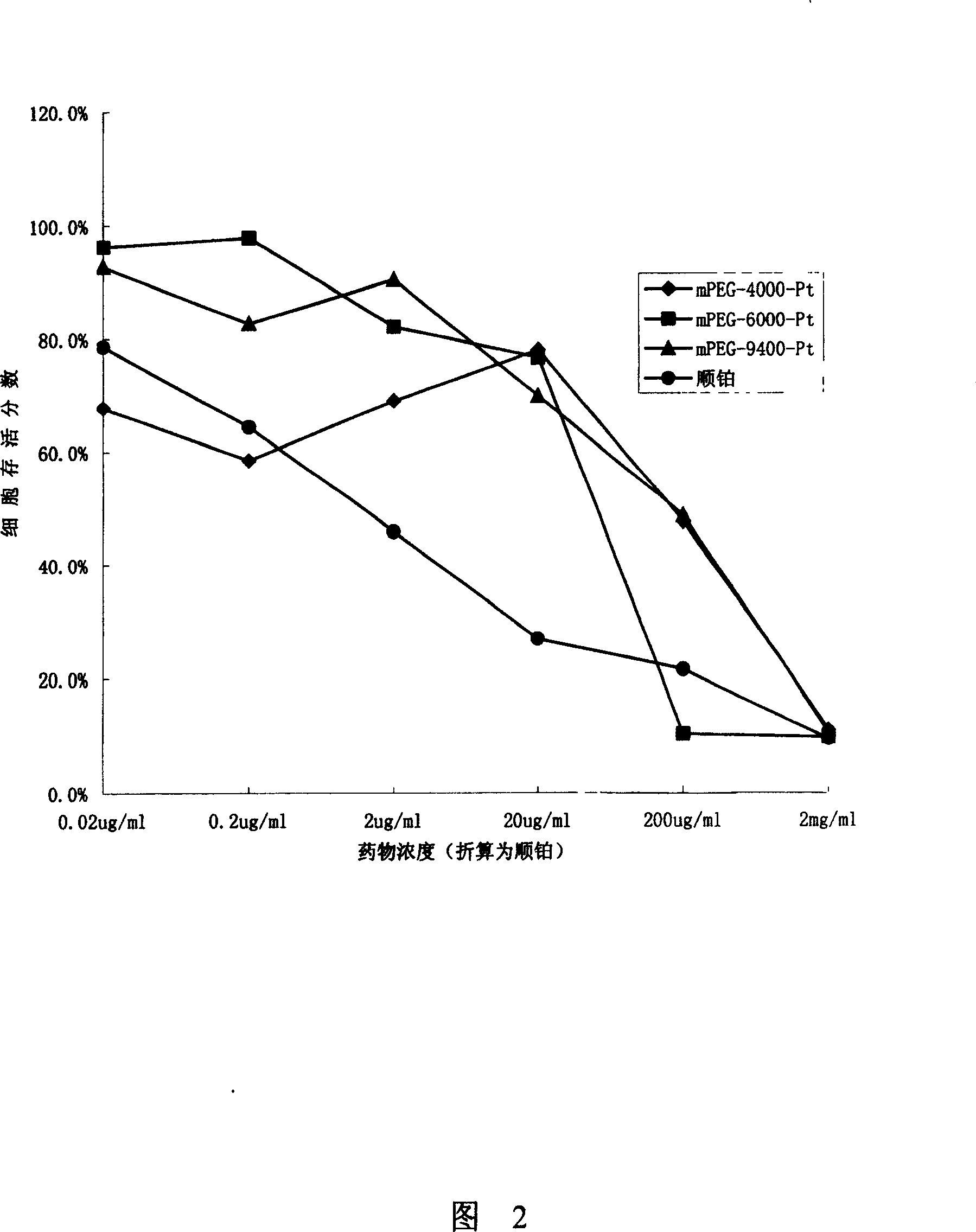
[0061] According to the steps of the above examples, the present invention synthesized polymer drugs with three molecular weights for in vitro cytotoxicity experiments. Three kinds of dual-arm mPEG (abbreviation: DImPEG) cisplatin conjugates with carrier molecular weights of 4000, 6000 and 9400 were synthesized: DImPEG-4000-Pt, DImPEG-6000-Pt, DImPEG-9400-Pt and three linear mPEG cisplatin Conjugates: mPEG-4000-Pt, mPEG-6000-Pt, mPEG-9400-Pt. The molar content of cisplatin in the above various medicines is listed in the following table 1:
[0062] Table.1 Molar content of platinum in macromolecule drugs
[0063] drug
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com