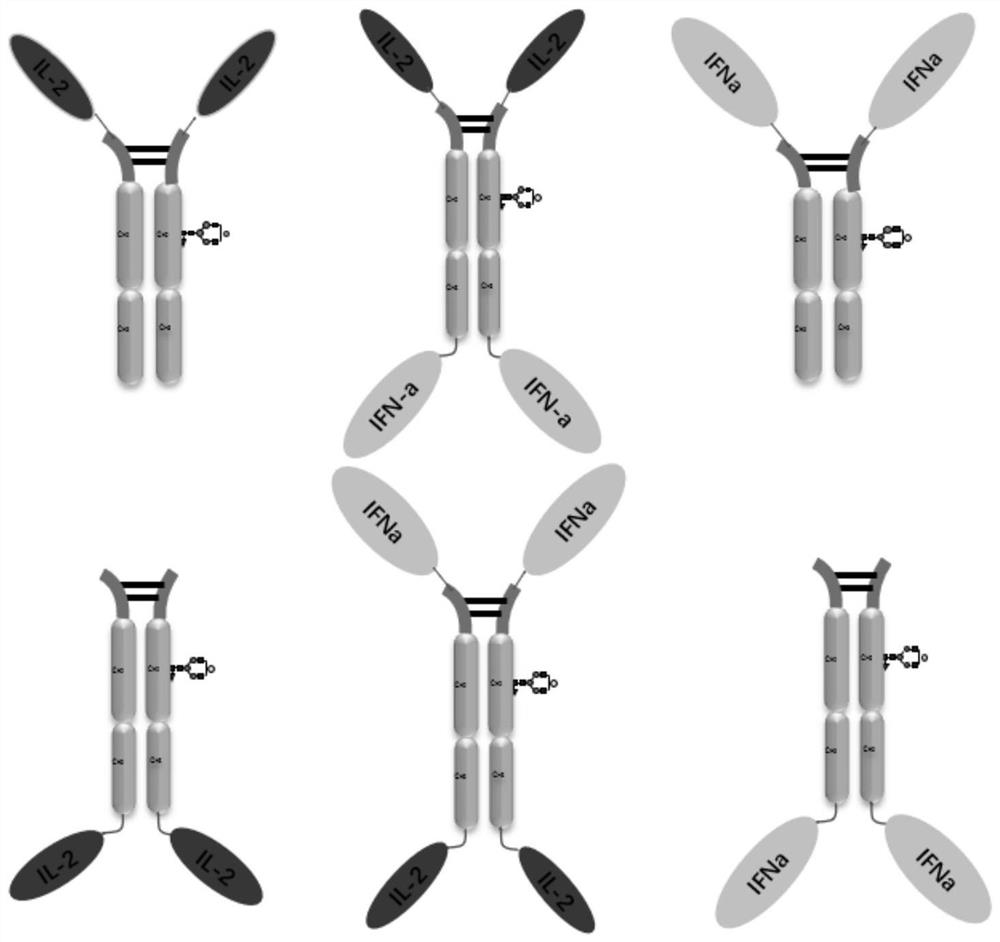
Design, preparation and application of novel IL-2, INF alpha and Fc fusion protein
A technology of IL-2 and fusion protein, applied in the field of biomedicine, can solve problems such as weak specificity, large side effects, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
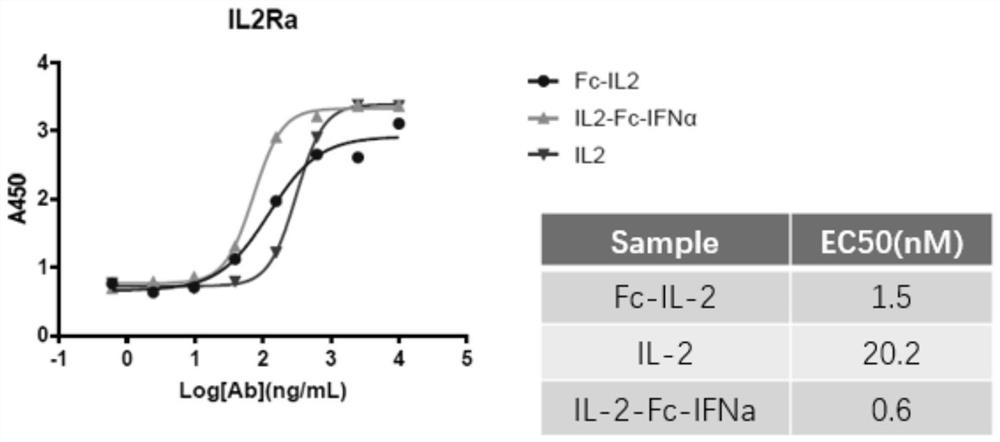
[0059] ELISA detection and receptor IL-2Rα protein binding ability
[0060]The packages are different (10000ng / ml, 2500ng / ml, 625ng / ml, 156.25ng / ml, 39.0625ng / ml, 9.765625ng / ml, 2.44140625ng / ml, 0). Laoshenzhou), 100UL / holes at 4 ° C; closed with 3 % skim milk powder at 37 ° C for 1h; add 1ug / ml fusion protein per hole and 100ul each of its control samples, and incubate 1h at 37 ° C; then add HRP -labeled sheep anti -human IL IL IL people IL. -2PAB, incubation at 37 ° C for 1h, after 10 minutes of color rendering, read OD450 on the enzyme label. Consequences figure 2 Essence
Embodiment 2
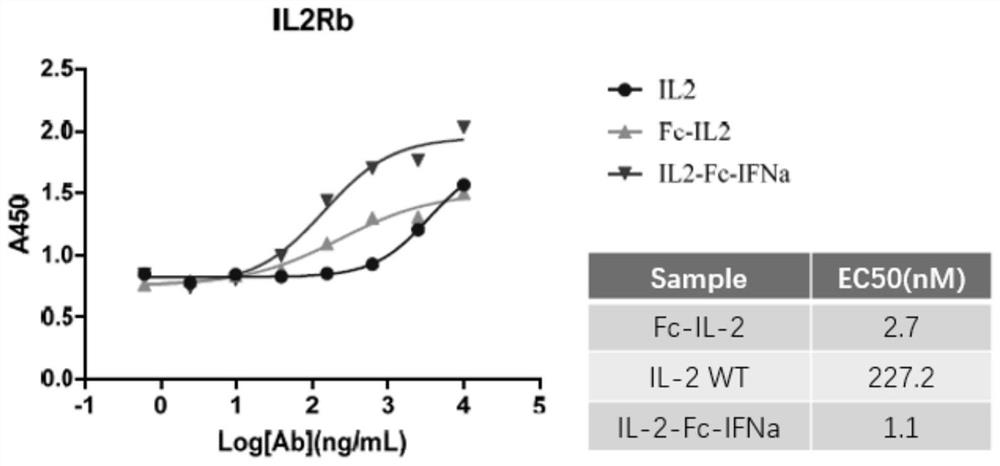
[0062] ELISA detection and receptor IL-2Rβ protein binding ability
[0063] The packages are different (10000ng / ml, 2500ng / ml, 625ng / ml, 156.25ng / ml, 39.0625ng / ml, 9.765625ng / ml, 2.44140625ng / ml, 0). Laoshenzhou), 100UL / holes at 4 ° C; closed with 3 % skim milk powder at 37 ° C for 1h; add 1ug / ml fusion protein per hole and 100ul each of its control samples, and incubate 1h at 37 ° C; then add HRP -labeled sheep anti -human IL IL IL people IL. -2PAB, incubation at 37 ° C for 1h, after 10 minutes of color rendering, read OD450 on the enzyme label. Consequences image 3 Essence
Embodiment 3
[0065] ELISA detection and receptor IFNαr2 protein binding ability
[0066] The packages are different concentrations (10000ng / ml, 2500ng / ml, 625ng / ml, 156.25ng / ml, 39.0625ng / ml, 9.765625ng / ml, 2.44140625ng / ml, 0) protein (10359-H02H, Yiliang Shenzhou ), 100UL / hole at 4 ° C overnight; closed 3 % skimmed milk powder at 37 ° C for 1h; add 1ug / ml fusion protein per hole and 100ul each of its control samples, incubate 1h 37 ° C; then add rabbit anti -human interferon 2,37 ℃ Incubation 1h, then add HRP -labeled sheep anti -rabbit H+L, incubate for 1h 37 ° C, and after 10 minutes of color rendering, read OD450 on the enzyme label. Consequences Figure 4 Essence
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com