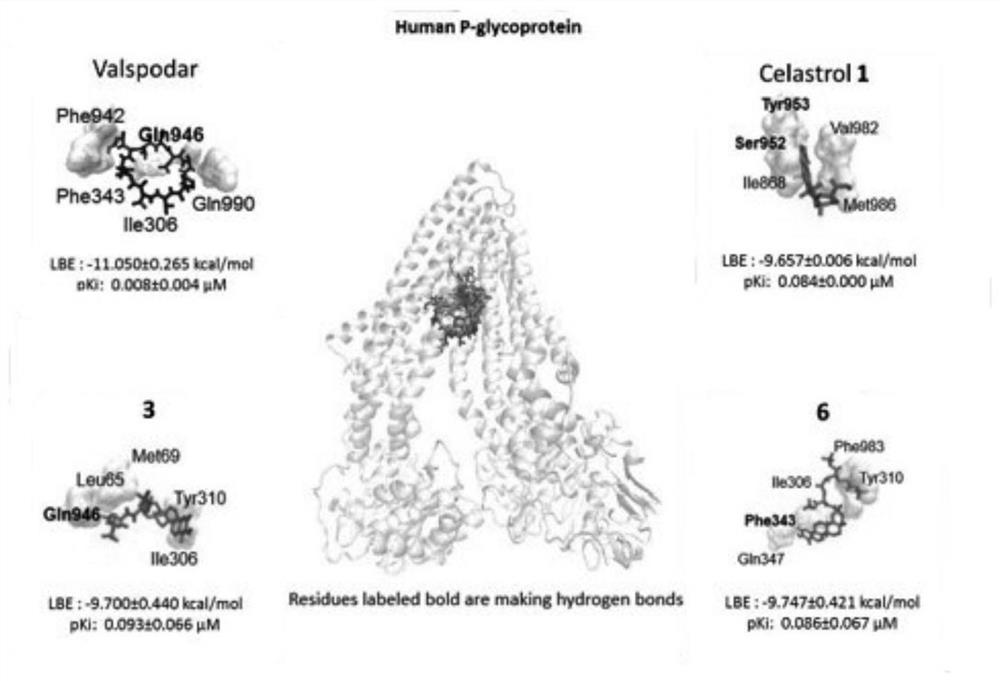
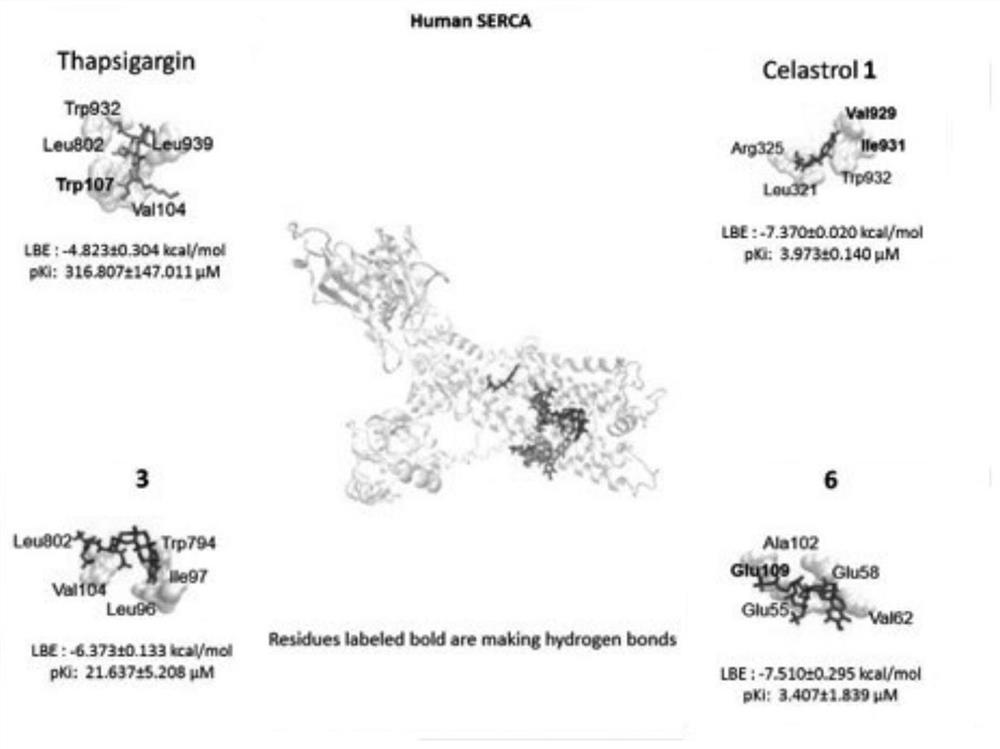
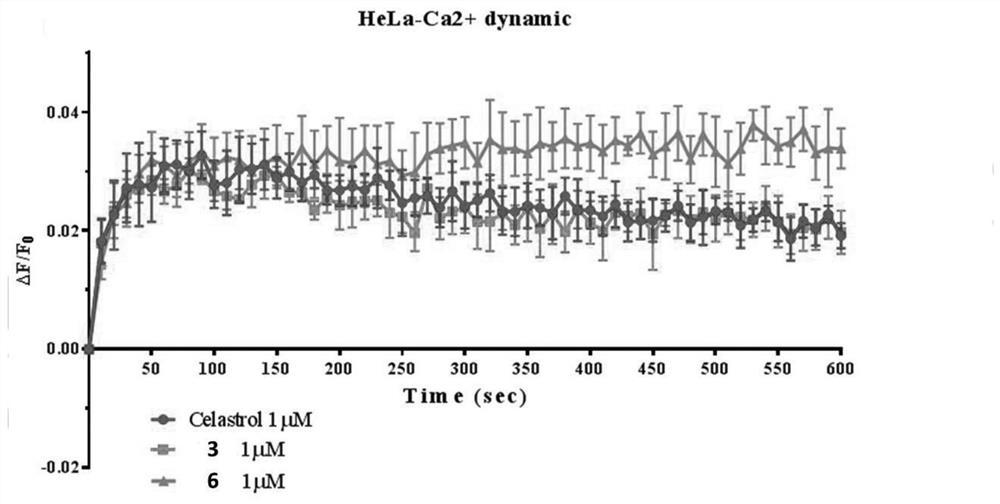
A kind of tripterine derivative and its preparation method and application
A technology of tripterine and its derivatives, which is applied in the field of medicine to achieve good anti-tumor effects, reduced toxicity, and improved selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Tripterylide (compound 1) was coupled with the dipeptide through the 29-position carboxylic acid of tripteryne in the form of an amide bond to obtain the following series of tripteryne derivatives (compounds 2-7):
[0035]
Embodiment 2
[0037] Tripterylide (compound 1) and tripterylide derivatives (compound 2-7) of Example 1 were tested for cytotoxicity by the MTT method, and the cells used were normal human liver cells LO2 and lung epithelial cells BEAS-2B, The test results are shown in Table 1:
[0038] Table 1
[0039]
[0040] It can be seen from Table 1 that compared with compound 1, the toxicity of compounds 2-7 to normal cells is significantly reduced, which proves that the structural modification of tripterine in the present invention effectively improves its performance.
Embodiment 3
[0042] By testing the selectivity index of tripterine derivatives (IC of normal cells 50 / IC of cancer cells 50 ), the result obtained is: compared with natural tripterine (compound 1), the selectivity of a series of tripterine derivatives in Example 1 to tumor cells is improved. Among them, the selection specificity of compound 3 to the liver cancer cell line HepAD38 was more than 30 times and more than 10 times higher than that of the normal cell line LO2 and CCD19Lu, respectively; The selectivity of the cell line HepAD38 and the liver cancer cell line HepG2 was improved by more than 12 times; the specific test data are shown in Table 2:
[0043] Table 2
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com