Polysubstituted triaryl macrocyclic compound and application thereof
A macrocyclic compound and triaryl technology, applied in the field of medicinal chemistry, can solve the problems of drug resistance in patients, and achieve the effects of strong plasticity, great transformation potential, and great development potential.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
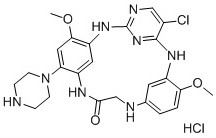
[0083] Example 1:3 5 -Chloro-1 6 ,5 6 -Dimethoxy-1 4 -(Piperidin-1-yl)-2,4,6,9-tetraaza-3(2,4)-pyrimidine-1,5(1,3)-dibenzocyclononan-8-one Hydrochloride (H-1)
[0084]
[0085] The synthetic route is as follows:
[0086]
[0087] Step 1. Synthesis of N-(3-((2,5-dichloropyrimidin-4-yl)amino)-4-methoxyphenyl)acetamide (3)
[0088] N-(3-Amino-4-methoxyphenyl)acetamide (1) (9 g, 50 mmol), 2,4,5-trichloropyrimidine (2) (10.23 g, 55 mmol), potassium phosphate (21.2 g, 100 mmol) and DMF (150 ml) were added to a 500 ml round-bottomed flask, under argon protection, the temperature was raised to 65 °C for reaction for 24 hours. After cooling, it was poured into 1L water for recrystallization to obtain a pale yellow solid, which was filtered and dried to obtain the product N-(3-((2,5-dichloropyrimidin-4-yl)amino)-4-methoxyphenyl)ethyl Amide (3) (pale yellow solid, 95% yield). 1H NMR (300 MHz, Chloroform- d ) δ 8.40 (s, 1H), 8.20 (t, J = 1.6 Hz, 1H), 8.14 (s, 1H), 7.59 (d...
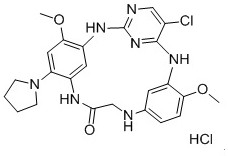
Embodiment 2
[0103] Example 2: 3 5 -chloro-1 6 ,5 6 -Dimethoxy-1 4 -(pyrrolidin-1-yl)-2,4,6,9-tetraaza-3(2,4)-pyrimidin-1,5(1,3)-dibenzocyclononan-8-one Hydrochloride (H-2)
[0104]
[0105] The synthetic route is as follows:
[0106]
[0107] Step 1. tert-butyl(3-((5-chloro-2-((2-methoxy-5-nitro-4-(pyrrolidin-1-yl)phenyl)amino)pyrimidin-4-yl )amino)-4-methoxyphenyl)carbamate (11) synthesis
[0108] Referring to the synthetic method of compound (8), the yield was 80%, red solid. 1 H NMR (400 MHz, Chloroform- d ) δ 8.81 – 8.68 (m, 1H), 8.34 (s, 1H), 8.11 – 8.01 (m, 1H), 7.91(d, J = 5.1 Hz, 1H), 7.26 (d, J = 5.1 Hz, 1H), 7.18 (d, J = 5.1 Hz, 1H), 6.87(d, J = 7.5 Hz, 1H), 6.84 – 6.76 (m, 1H), 6.36 – 6.28 (m, 1H), 3.94 (d, J =8.1 Hz, 3H), 3.91 (t, J = 4.8 Hz, 3H), 3.31 – 3.20 (m, 4H), 2.03 – 1.94 (m,4H), 1.53 – 1.43 (m, 9H).
[0109] Step 2. tert-Butyl (3-((2-((5-amino-2-methoxy-4-(pyrrolidin-1-yl)phenyl)amino)-5-chloropyrimidin-4-yl) Synthesis of amino)-4-methoxyphe...
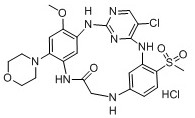
Embodiment 3
[0115] Example 3: 3 5 -chloro-1 6 ,5 6 -Dimethoxy-1 4 -(pyrrolidin-1-yl)-2,4,6,9-tetraaza-3(2,4)-pyrimidin-1,5(1,3)-dibenzocyclononan-8-one Hydrochloride (H-3)
[0116]
[0117] The synthetic route is as follows:
[0118]
[0119] Step 1. tert-butyl(3-((5-chloro-2-((2-methoxy-4-morpholine-5-nitrophenyl)amino)pyrimidin-4-yl)amino)-4- Synthesis of methoxyphenyl) carbamate (14)
[0120] Referring to the synthetic method of compound (8), the yield was 80%, red solid. 1 H NMR (400 MHz, DMSO- d 6 ) δ9.07 (s, 1H), 8.57 (s, 1H), 8.21 (d, J = 10.7 Hz, 3H), 7.82 (s, 1H), 7.34 –7.27 (m, 1H), 6.96 (d, J = 9.0 Hz, 1H), 6.84 (s, 1H), 3.97 (s, 3H), 3.79 (s,3H), 3.74 – 3.70 (m, 4H), 3.02 (d, J = 4.5 Hz, 4H), 1.48 (s, 9H).
[0121] Step 2. tert-Butyl(3-((2-((5-amino-2-methoxy-4-morpholinephenyl)amino)-5-chloropyrimidin-4-yl)amino)-4-methanol Synthesis of oxyphenyl) carbamate (15)
[0122] Referring to the synthetic method of compound (8), the yield is 80%, white solid. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com