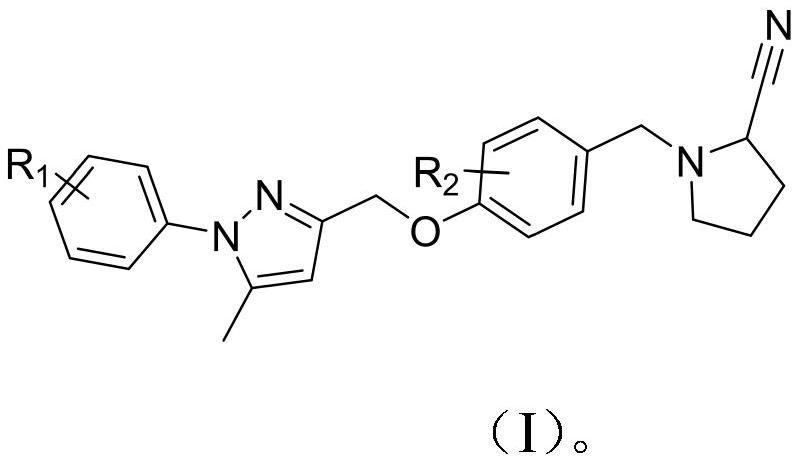
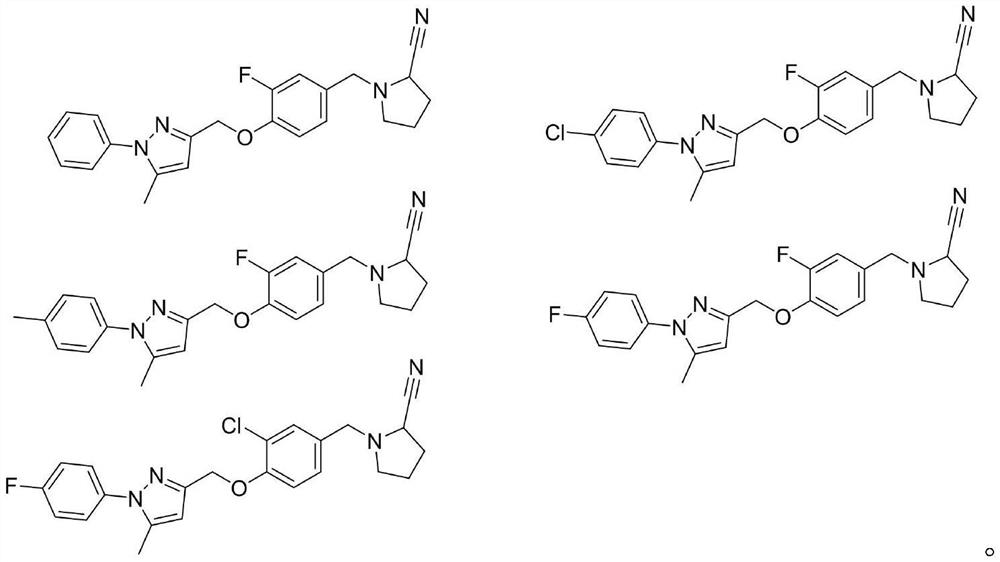
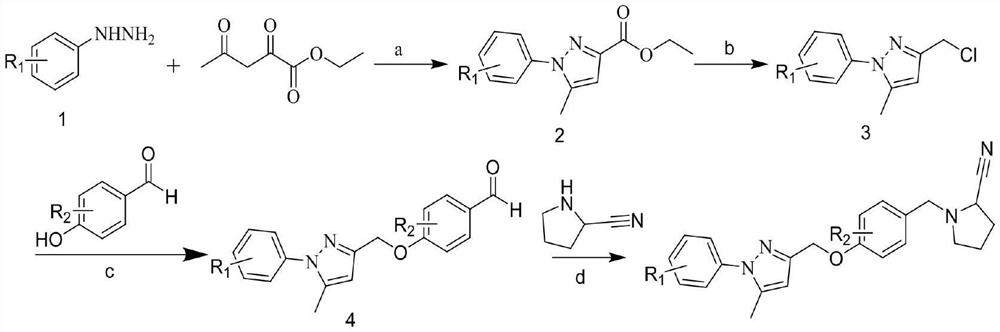
Phenyl-1h-pyrazole derivatives and their application in antitumor drugs
A technology of pyrazoles and derivatives, which is applied in the field of new phenyl-1H-pyrazole derivatives, can solve the problems of small molecule drugs on the market, and achieve the effect of novel skeleton, good activity and great research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] (1) Synthesis of ethyl 5-methyl-1-phenyl-1H-pyrazole-3-carboxylate
[0026] Dissolve 2.0 g (13.83 mmol) of phenylhydrazine hydrochloride and 1.47 g (9.22 mmol) of ethyl acetylacetonate in ethanol, and raise the temperature to reflux for 6 hours. TLC detected that the reaction was complete, and concentrated under reduced pressure to distill off the solvent. Then add water, extract with ethyl acetate, wash the organic layer with saturated brine, Na 2 SO 4 Let dry overnight. The desiccant was filtered off, the solvent was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography to obtain 1.62 g of white solid with a yield of 75.3%. 1 H NMR (400MHz, DMSO-d 6 )δ: 7.59~7.54(m,5H),6.73(s,1H),4.32(q,J=7.1Hz,2H),2.35(s,3H),1.31(t,J=7.1Hz,3H).
[0027] (2) Synthesis of 3-chloromethyl-5-methyl-1-phenyl-1H-pyrazole
[0028] The intermediate 5-methyl-1-phenyl-1H-pyrazole-3-carboxylic acid ethyl ester (1.5g, 6.51mmol) wa...
Embodiment 2
[0035]
[0036] 1H-NMR (600MHz, DMSO-d 6 )δ7.58(d,J=7.5Hz,2H),7.36(d,J=7.4Hz,2H),7.01–6.84(m,3H),6.64(s,1H),5.21(s,2H), 3.61(s,2H),3.32(t,J=7.1Hz,1H),2.44–2.34(m,2H),2.32(s,3H),1.96–1.63(m,4H).
Embodiment 3
[0038]
[0039] 1 H-NMR (600MHz, DMSO-d 6 )δ7.52(d,J=7.4Hz,2H),7.26(d,J=7.4Hz,2H),6.97–6.84(m,3H),6.66(s,1H),5.22(s,2H), 3.62(s,2H),3.36(t,J=7.1Hz,1H),2.48(s,3H),2.45–2.34(m,2H),2.32(s,3H),1.98–1.61(m,4H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com