An H3 receptor antagonist combined with a cholinesterase inhibitor for use in the treatment of alzheimer's disease
A technology for Alzheimer's and inhibitors, applied in drug combinations, nervous system diseases, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1: Preclinical Research
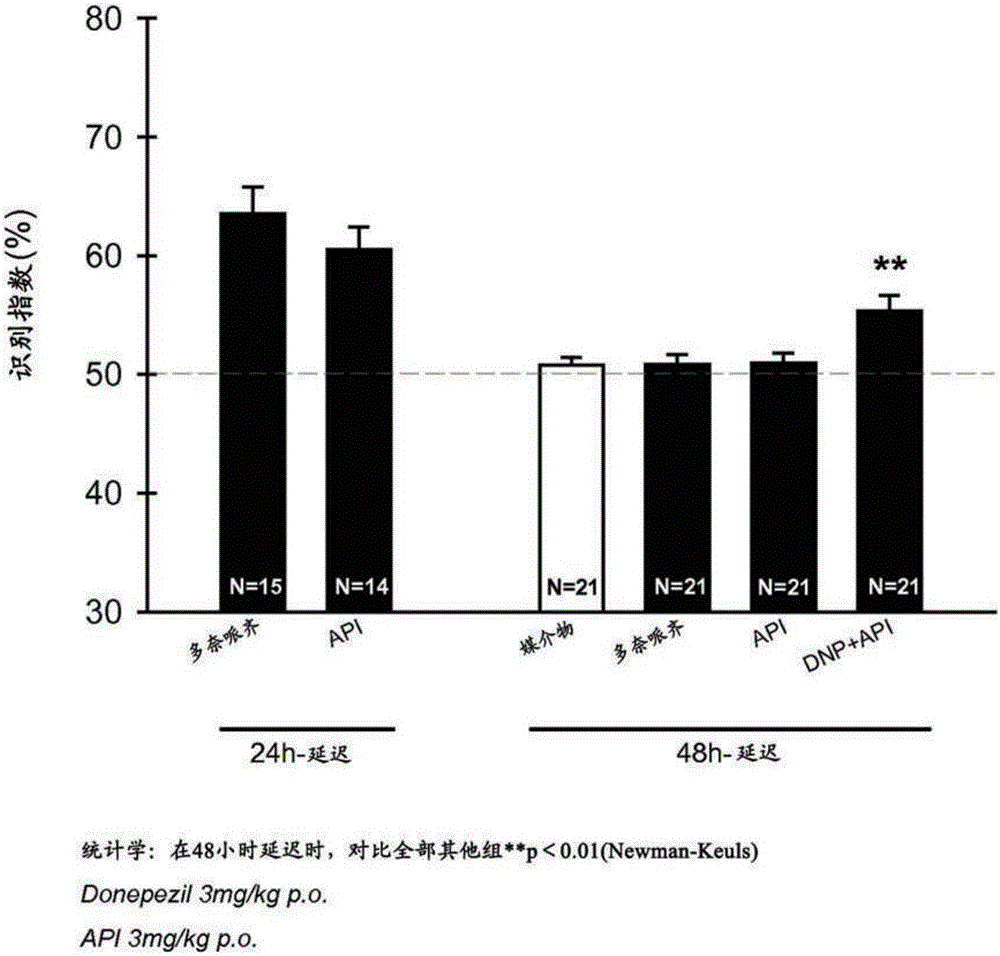
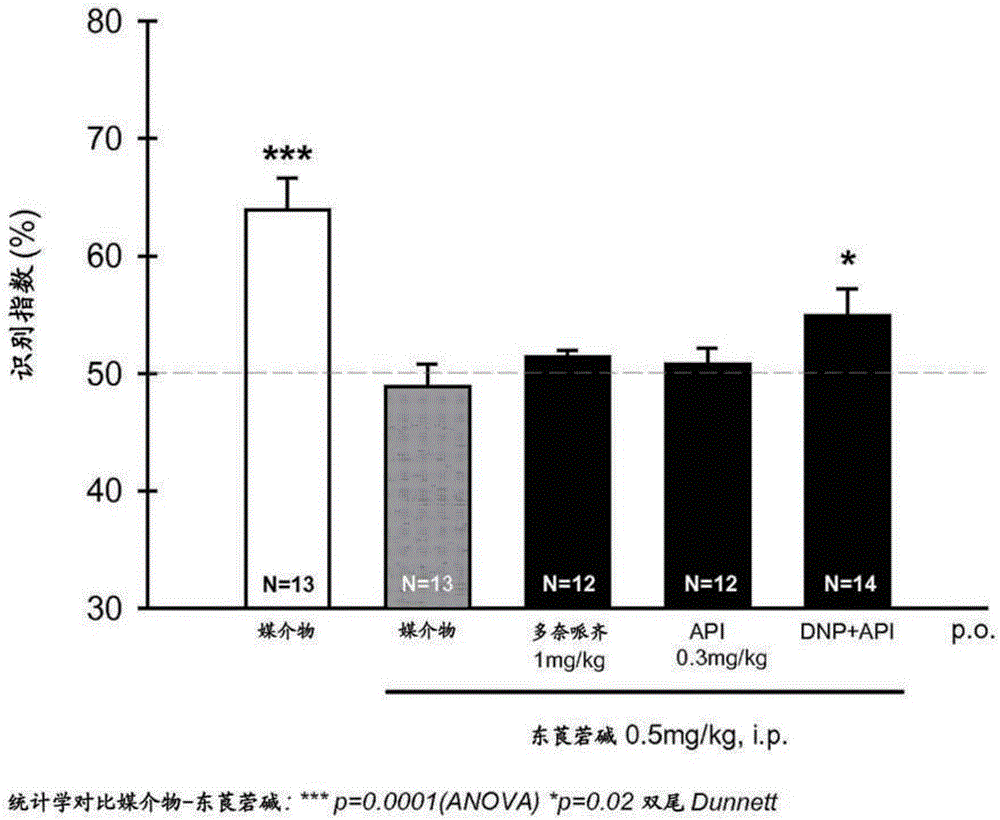
[0099]In the object recognition test, 2-(cyclohexylmethyl)-N-{2-[(2S)-1-methylpyrrolidin-2-yl]ethyl}-1,2,3,4-tetrahydro Oral administration of isoquinoline-7-sulfa hydrogen fumarate monohydrate (3 mg / kg) or donepezil hydrochloride (DNP) (3 mg / kg), respectively, enhanced memory in CD1 male mice 24 hours after administration , in contrast to the lack of cognitive-promoting effects observed 48 hours after administration of either molecule. However, 3 mg / kg of 2-(cyclohexylmethyl)-N-{2-[(2S)-1-methylpyrrolidin-2-yl]ethyl}-1,2,3,4-tetrahydro The co-administration of isoquinoline-7-sulfa hydrogen fumarate monohydrate and 3 mg / kg donepezil hydrochloride had a significant procognitive effect 48 hours after administration. The cognitive-promoting effect of this co-administration at 48 hours indicated that 2-(cyclohexylmethyl)-N-{2-[(2S)-1-methylpyrrolidin-2-yl]ethyl}-1 , Potentiation effect of 2,3,4-tetrahydroisoquinoline-7-sulfa hydrogen fum...
Embodiment 2
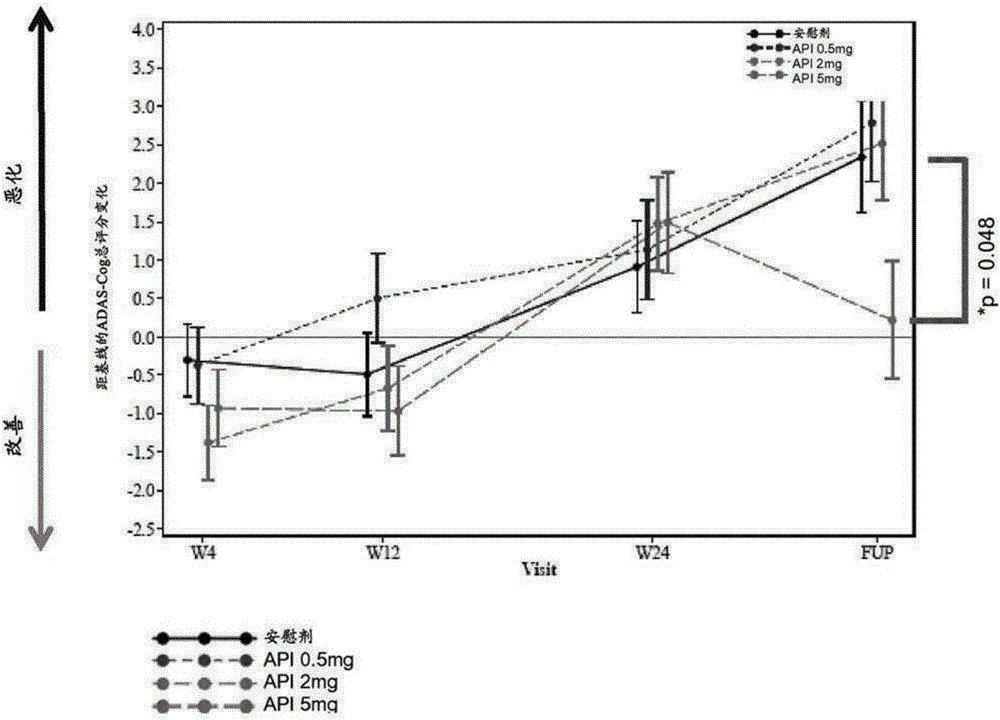
[0101] Embodiment 2: clinical research
[0102] A randomized, double-blind, parallel-group, placebo-controlled clinical study was carried out to investigate the Efficacy, safety and tolerability of 1,2,3,4-tetrahydroisoquinoline-7-sulfonamide hydrogen fumarate monohydrate as adjunctive therapy to donepezil on cognitive performance.
[0103] More specifically, patients with mild to moderate Alzheimer's disease receiving stable donepezil therapy were randomized to receive 2-(cyclohexylmethyl)-N- {2-[(2S)-1-Methylpyrrolidin-2-yl]ethyl}-1,2,3,4-tetrahydroisoquinoline-7-sulfonamide hydrogen fumarate monohydrate or placebo dose for 24 weeks, and a follow-up period of 10 weeks.
[0104] 291 patients (290 treated) participated in the study. The baseline characteristics of the randomized patients are described in Table 1.
[0105] Table 1: Randomized populations
[0106]
[0107]
[0108] The modified intention-to-treat (mITT) population were randomized patients who received...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com