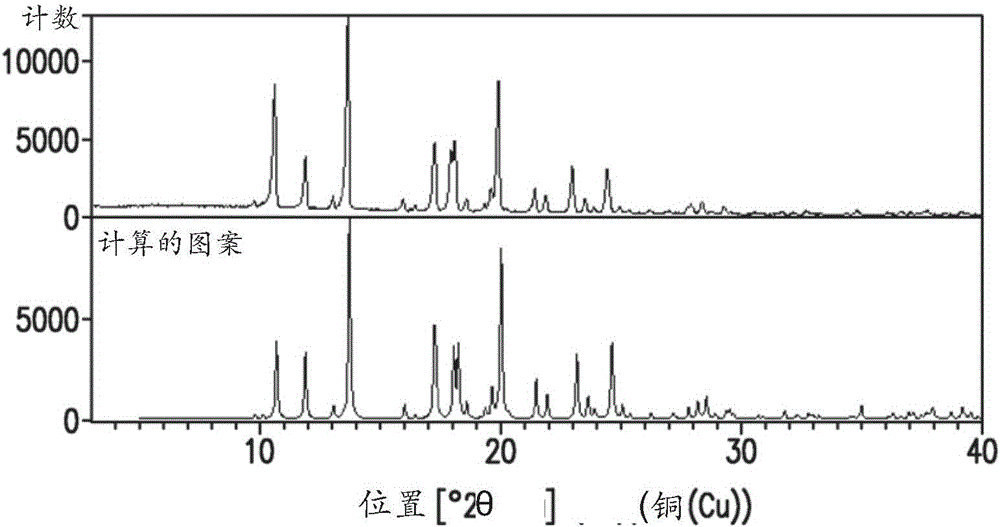
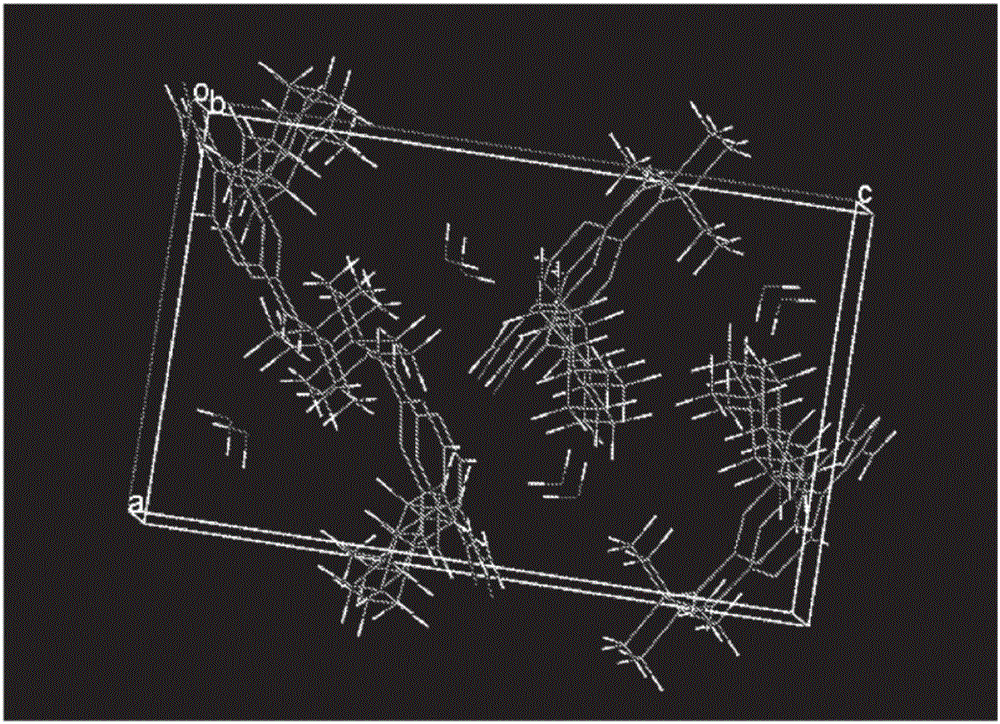
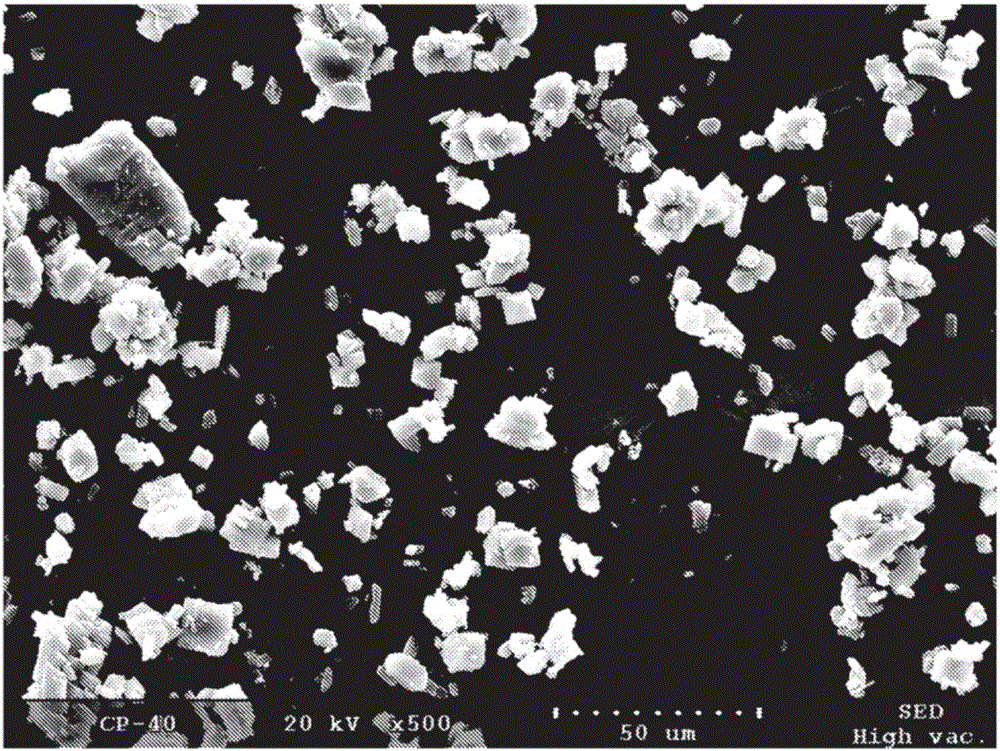
Solid forms of 2-(tert-butylamino)-4-((1r,3r,4r)-3-hydroxy-4-methylcyclohexylamino)-pyrimidine-5-carboxamide, compositions thereof and methods of their use
A form, solid technology, applied in 2-(tert-butylamino)-4-((1R, can solve problems such as complex determination and selection of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0858] Example 1: 2-(tert-butylamino)-4-{[(1R,3R,4R)-3-hydroxy-4-methylcyclohexyl]amino}pyrimidine-5-carboxamide
[0859]
[0860] 2-Chloro-4-{[(1R,3R,4R)-3-hydroxy-4-methylcyclohexyl]amino}pyrimidine-5-carboxamide: Add (1R,2R, 5R)-5-amino-2-methylcyclohexanol hydrochloride (16.0kg), 2,4-dichloropyrimidine-5-carboxamide (19.0kg), K 2 CO 3 (14.9kg) and THF (160L). The batch was cooled to 0 °C and water (160 L) was added. Stirring of the batch was continued at 0°C for 1 hour and the temperature was raised to 25°C and held for 16 hours. Water (288 L) was added to the batch while maintaining the batch at 25°C, the batch was cooled to 15°C and stirring was continued for 4 hours. The batch was filtered, rinsed twice with water (2 x 80 L), and dried in a vacuum oven at 40 °C under a nitrogen purge for 24 hours to give 2-chloro-4-{[(1R,3R,4R)-3 -Hydroxy-4-methylcyclohexyl]amino}pyrimidine-5-carboxamide as a white powder (23.3 kg, yield 86%). 1 H NMR (DMSO-d 6 )δ0.93(d, J=5.7...
Embodiment 2
[0863]Example 2: 4-(tert-butylamino)-2-((trans-4-hydroxycyclohexyl)amino)pyrimidine-5-carboxamide
[0864]
[0865] 4-(tert-butylamino)-2-chloropyrimidine-5-carboxamide: Stir 2,4-dichloro-pyrimidine-5-carboxamide (10.0g), DIPEA (11mL) in NMP (30mL ) in the mixture. Tert-butylamine (6.6 mL) was charged to the mixture, and the mixture was stirred at 25°C for 16 hr. Water (100 mL) was added to the mixture at 25°C. The mixture was stirred for 1 hour. The suspension was filtered, washed with water (50 mL) and dried in a vacuum oven at 40 °C under nitrogen purge for 24 hours to give 4-(tert-butylamino)-2-chloropyrimidine-5-carboxamide as a white solid (8.7 g, 84%). 1 H NMR (DMSO-d 6 ) δ 9.41 (s, 1H), 8.55 (s, 1H), 8.19 (s, 1H), 7.67 (s, 1H), 1.42 (s, 9H).
[0866] 4-(tert-butylamino)-2-((trans-4-hydroxycyclohexyl)amino)pyrimidine-5-carboxamide: 4-(tert-butylamino)-2-chloropyrimidine-5-carboxamide (0.5g), trans-4-aminocyclohexanol hydrochloride (0.40g), Na 2 CO 3 (0.28 g)...
Embodiment 3
[0868] Example 3: 4-(bicyclo[1.1.1]pent-1-ylamino)-2-(((1R,3S)-3-hydroxycyclohexyl)amino)pyrimidine-5-carboxamide
[0869]
[0870] 4-(bicyclo[1.1.1]pent-1-ylamino)-2-chloropyrimidine-5-carboxamide: Stir 2,4-dichloro-pyrimidine-5-carboxamide (2 g) at 25°C, A mixture of bicyclo[1.1.1]pentan-1-amine hydrochloride (1.18 g), sodium bicarbonate (1.75 g) and NMP (10 mL) for 24 hours. Water (10 mL) was charged and the reaction temperature was maintained below 30°C, and the mixture was stirred at 25°C for 2 hours. The suspension was filtered and washed with NMP:water (1:1 10 mL), then water (2×10 mL), and dried in a vacuum oven at 40° C. under a nitrogen purge to give 4-(bicyclo[1.1.1]penta- 1-ylamino)-2-chloropyrimidine-5-carboxamide as a white solid (1.97 g, 83% yield). 1 H NMR (DMSO-d 6 )δ2.14(s, 6H), 2.51-2.53(m, 1H), 7.76(br.s., 1H), 8.23(br.s., 1H), 8.60(s, 1H), 9.57(s, 1H).
[0871] 4-(bicyclo[1.1.1]pent-1-ylamino)-2-(((1R,3S)-3-hydroxycyclohexyl)amino)pyrimidine-5-carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com