Binuclear ferrocene hexacyanoferrate and preparation method thereof
A technology of ferrocene hexacyano, potassium hexacyanoferrate, applied in the field of binuclear ferrocene hexacyanoferrate and preparation thereof, can solve problems such as non-volatile, achieve non-volatile, strong migration resistance, The effect of increasing iron content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
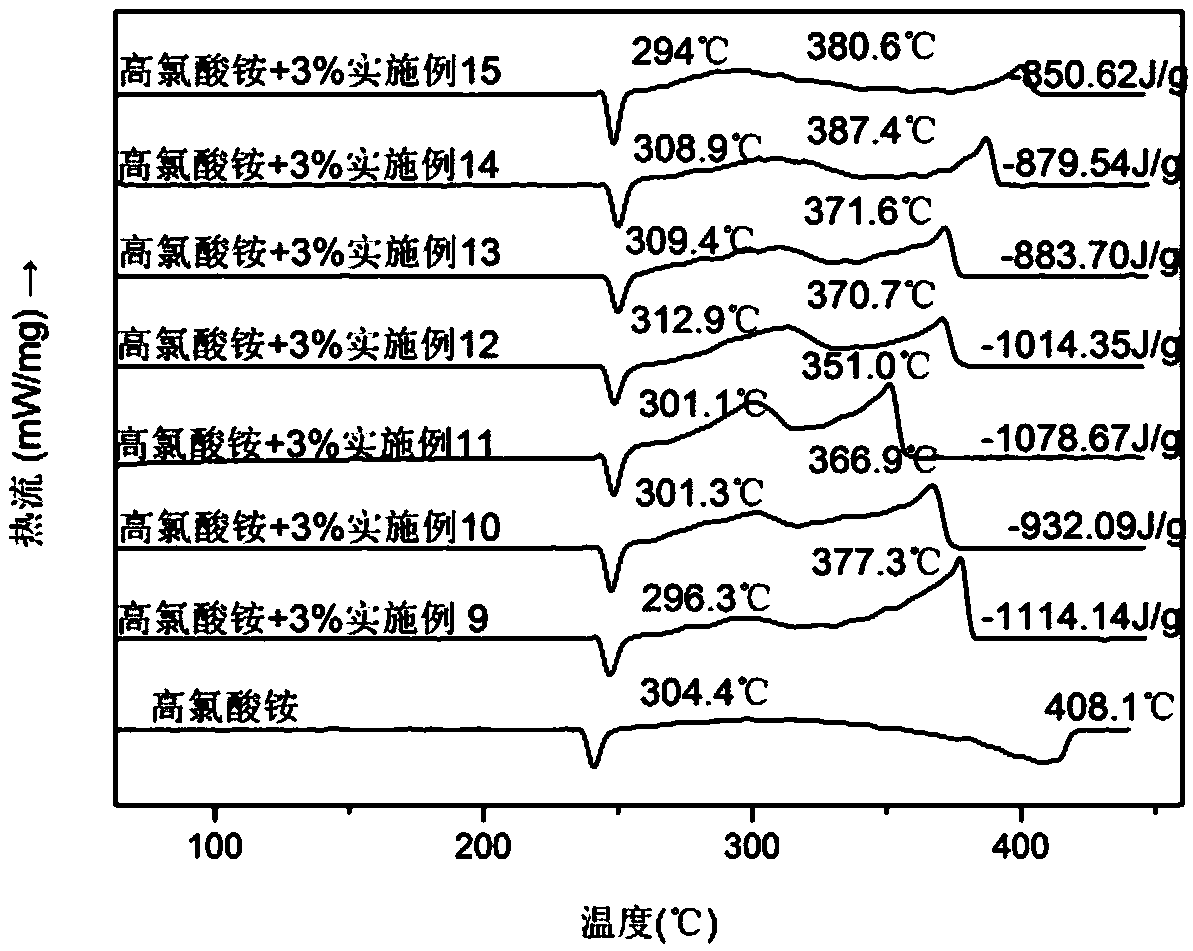
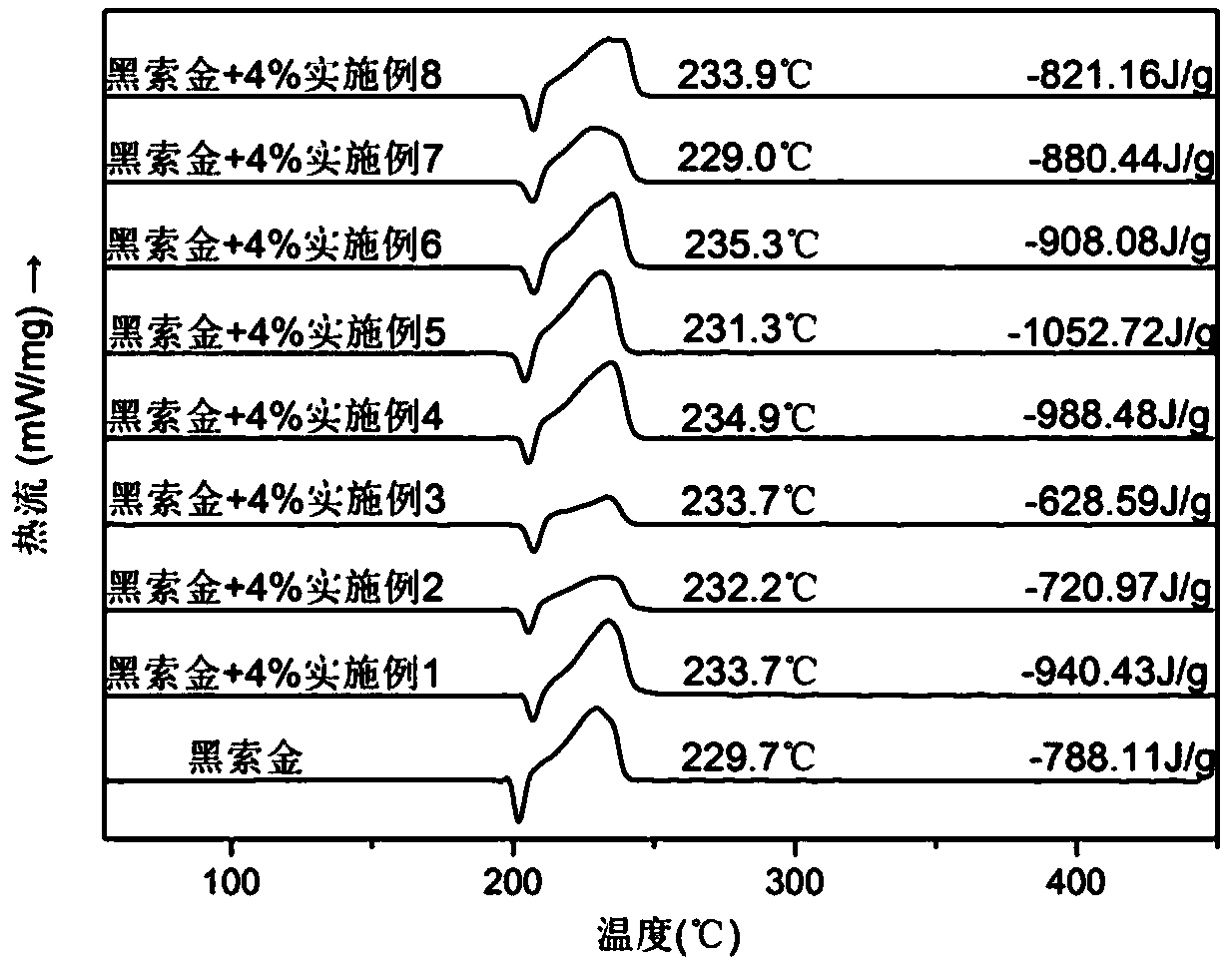
Image
Examples
Embodiment 1
[0029] Preparation of hexacyanoferrate of 1,3-propylene bis[(1-ferrocenylmethyl)dimethylammonium with the following structural formula, the specific preparation method is:
[0030]
[0031] Dissolve 0.076g (0.8mmol) potassium hexacyanoferrate completely in a 50mL round bottom flask containing 20mL of distilled water. After the solution is completely transparent, add the transparent solution dropwise to 5mL to dissolve 0.478g (1mmol) of 1 , In the methanol solution of 3-propylene bis[(1-ferrocenylmethyl)dimethylammonium iodide, a dark blue precipitate was formed immediately, stirred and reacted at room temperature for 4 hours, filtered, and the filter cake was washed with distilled water for 3 and then dried in a vacuum oven at 45°C for 48 hours to obtain hexacyanoferrate of dark blue solid powder 1,3-propylenebis[(1-ferrocenylmethyl)dimethylammonium 0.566 g, the yield is 85%.
[0032] The spectral data of the product is: IR (cm -1 ): 3012 (C=C-H), 2998 (-CH 3 ), 2864 (-C...
Embodiment 2
[0037] Preparation of hexacyanoferrate of 1,4-butylene bis[(1-ferrocenylmethyl)dimethylammonium with the following structural formula, the specific preparation method is:
[0038]
[0039] Dissolve 0.076g (0.8mmol) potassium hexacyanoferrate completely in a 50mL round bottom flask containing 20mL of distilled water. After the solution is completely transparent, add the transparent solution dropwise to 5mL to dissolve 0.492g (1mmol) of 1 , In the methanol solution of 4-butylenebis[(1-ferrocenylmethyl)dimethylammonium iodide, a black precipitate was formed immediately, stirred and reacted at room temperature for 4 hours, filtered, and the filter cake was washed 3 times with distilled water, Then place it in a vacuum oven and dry it at 45° C. for 48 hours to obtain 0.592 g of hexacyanoferrate of black solid powder 1,4-butylene bis[(1-ferrocenylmethyl) dimethyl ammonium, which The yield was 87%.
[0040] The spectral data of the product is: IR (cm -1 ): 3063 (C=C-H), 2919 (-C...
Embodiment 3
[0045] Preparation of hexacyanoferrate of 1,5-pentylidene bis[(1-ferrocenylmethyl)dimethylammonium with the following structural formula, the specific preparation method is:
[0046]
[0047] Dissolve 0.076g (0.8mmol) potassium hexacyanoferrate completely in a 50mL round bottom flask containing 20mL of distilled water. After the solution is completely transparent, add the transparent solution dropwise to 5mL to dissolve 0.506g (1mmol) of 1 , In the methanol solution of 5-pentylidene bis[(1-ferrocenylmethyl) dimethyl ammonium iodide, a yellow-green precipitate is generated immediately, and other steps are the same as in Example 1 to obtain a yellow-green solid powder 1 , 0.597 g of hexacyanoferrate of 5-pentylidene bis[(1-ferrocenylmethyl)dimethylammonium, the yield was 86%.
[0048] The spectral data of the product is: IR (cm -1 ): 3057 (C=C-H), 2966 (-CH 3 ), 2881 (-CH 2 ), 2112 (C≡N), 1632 (C=C). Elemental analysis (theoretical calculation values in brackets): C% 59...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat release | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com