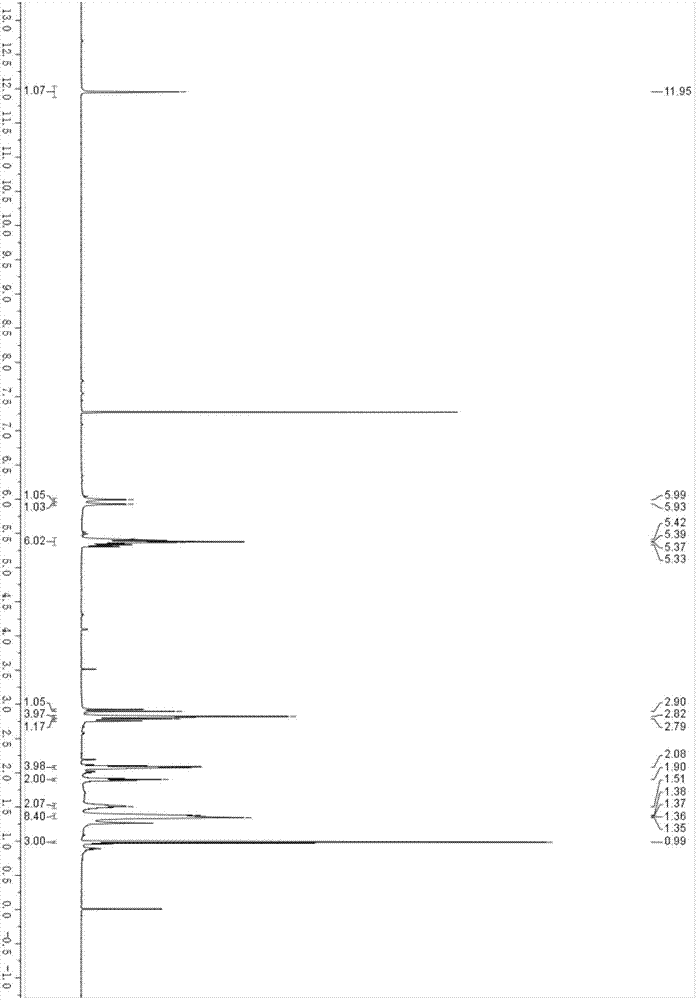
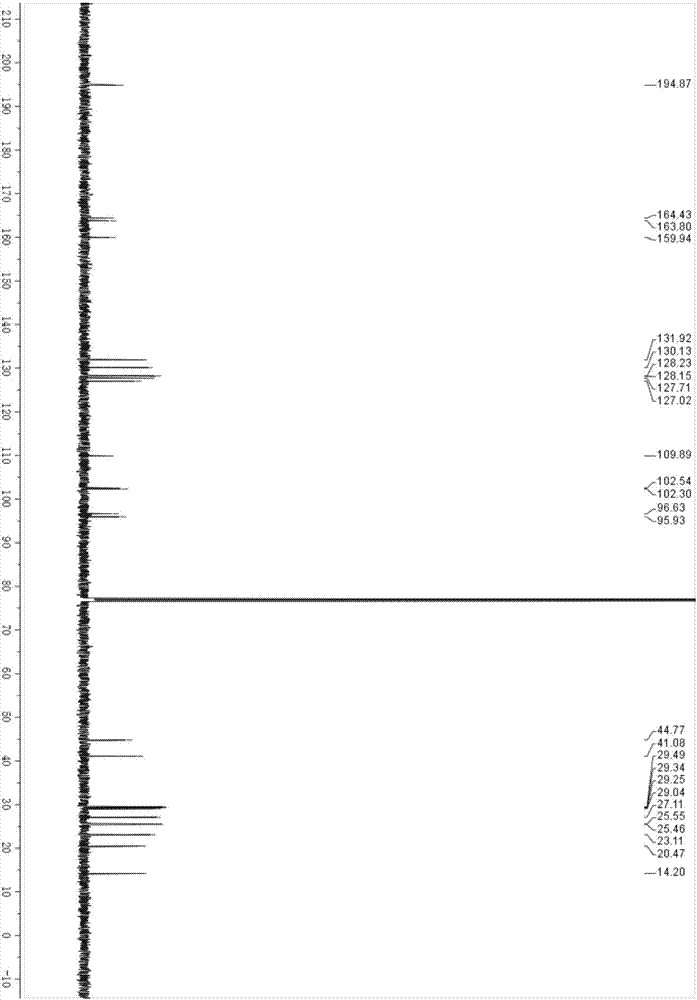
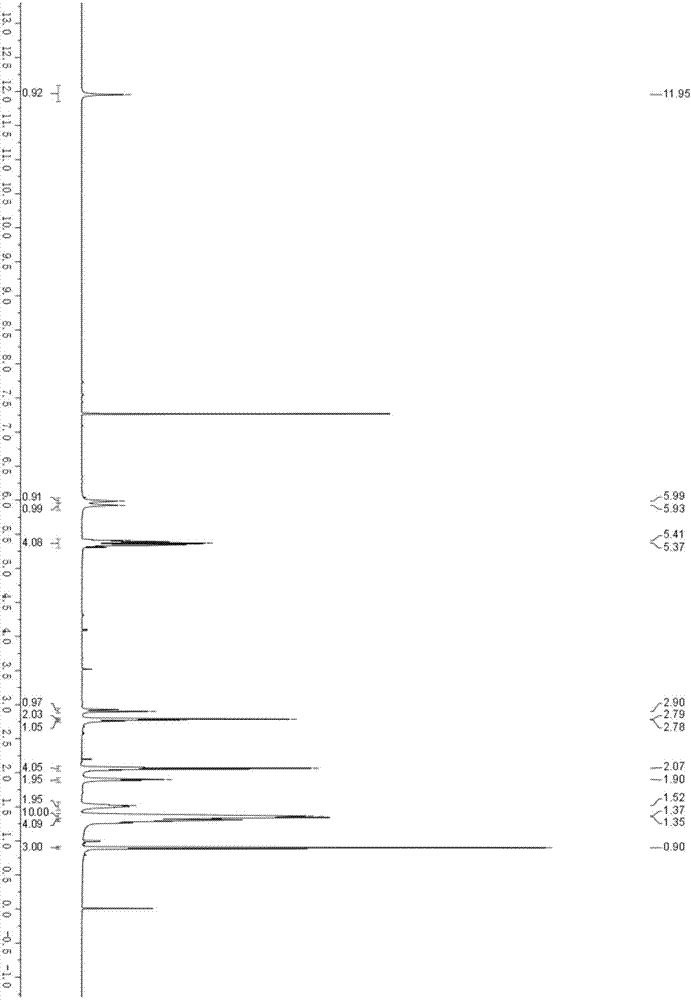
Phloroglucinol derivative capable of inhibiting PTP1B activity, and preparation method and applications thereof
A technology of phloroglucinol and inhibitory activity, which is applied in the field of phloroglucinol derivatives that can inhibit PTP1B activity and its preparation and application, and can solve problems such as type 2 diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of acylphloroglucinol derivatives with fatty side chains
[0037] 1. Preparation of black ink seed extract extract
[0038] (1) Preparation of extract
[0039] The black ink seeds (5kg) were pulverized, and the pulverized seed powder was cold-soaked for 24 hours with petroleum ether 10L, filtered, and this step was repeated 2 times. The petroleum ether extract was discarded, and the filter residue was cold-soaked in 10 L of 70% acetone-water solution for 4 times, each time for 24 hours, filtered, and the filtrates were combined to obtain the black black seed extract.
[0040] (2) Preparation of extract extract
[0041] The above extract was concentrated under reduced pressure at a temperature of ≤40°C to remove acetone and water to obtain 1200 g of crude extract.
[0042] 2. Separation and purification
[0043] (1) Disperse the above-mentioned 70% acetone-water extract in 2.5L water to make a suspension, and extract the suspension 4 times with ethyl acet...
Embodiment 2
[0080] PTP1B inhibitory activity assay
[0081] 1 Experimental principle and method
[0082] (1) The activity of PTP1B was determined based on the principle that the product of PTP1B hydrolyzing one phosphate group of p-nitrophenylphosphate (p-NPP) has absorption at a wavelength of 405 nm.
[0083] (2) 100μL reaction system: p-NPP 2mM; 1.15μg / mL GST-PTP1B1-321 protein solution; sample or DMSO; buffer [50mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH7. 3; 100 mM NaCl; 0.1% bovine serum albumin (BSA); 1 mM dithiothreitol (DTT)].
[0084] (3) Experimental method: Add 1 μL of PTP1B protein solution (0.115 mg / mL), 1 μL of test sample or positive control sample or 1 μL of DMSO, and 96 μL of buffer solution in a 96-well plate, mix well, and incubate at 30°C for 10 minutes. Then 2 μL of p-NPP (20 mM) was added to react for 30 min, and finally 3M NAOH was added to terminate the reaction, and the absorbance was measured at 405 nm using a SpectraMax MD5 microplate re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com