A kind of hemostatic material and preparation method thereof
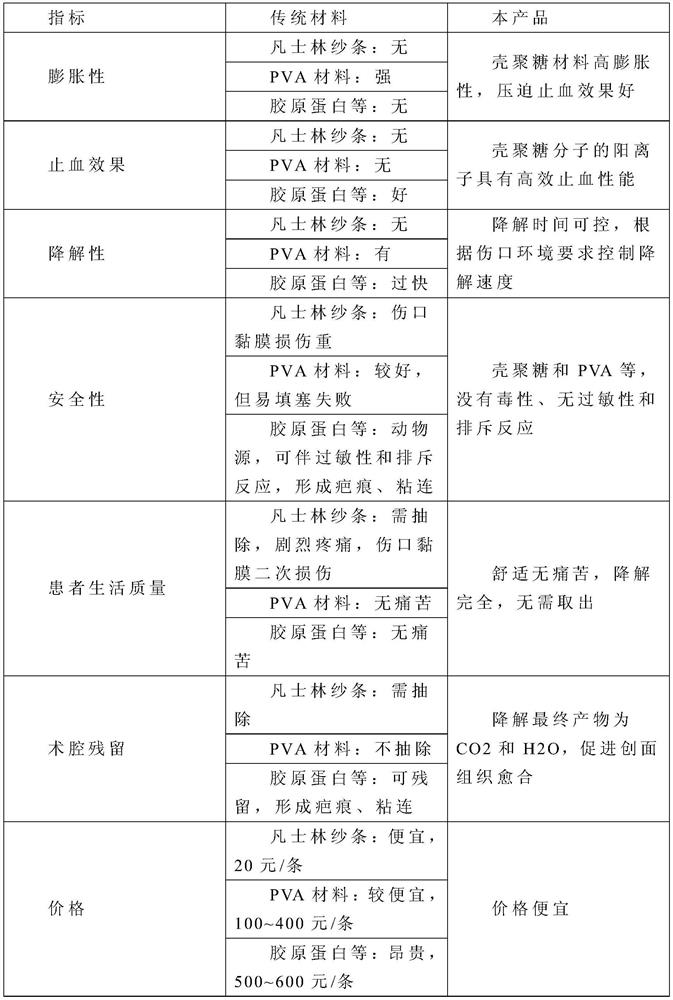
A technology for hemostatic materials and raw materials, which is applied in pharmaceutical formulations, bandages, drug delivery and other directions, can solve problems such as performance to be improved, failure of packing hemostasis, limited packing pressure, etc., and achieves good solubility and biodegradability, good extrusion hemostasis, The effect of good permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] In the second aspect, the present invention provides a method for preparing a hemostatic material; the preparation method can be realized through the following two different operations.
[0047] The first preparation method comprises the following steps in turn:
[0048] (1) Dissolving: Carboxymethyl chitosan and polyvinyl alcohol are dissolved in water according to the above ratio to obtain the main raw material aqueous solution (mass percentage concentration is 0.5-5%, can be 0.5%, 1%, 2%, 3% %, 4%, 5%)
[0049] (2) Cross-linking: According to the above ratio, add a compound toughener and an antibacterial agent mixed with polyethylene glycol and glycerin to the aqueous solution of the main raw materials to carry out a cross-linking reaction to obtain a cross-linked product.
[0050] Wherein, the mixing system composed of the main raw material aqueous solution, compound toughening material and antibacterial agent is stirred for 15-40min (preferably 30min); , its mass...
Embodiment 1
[0074] The hemostatic material of this embodiment is prepared by the first method, and the hemostatic material includes the following raw materials in parts by weight:
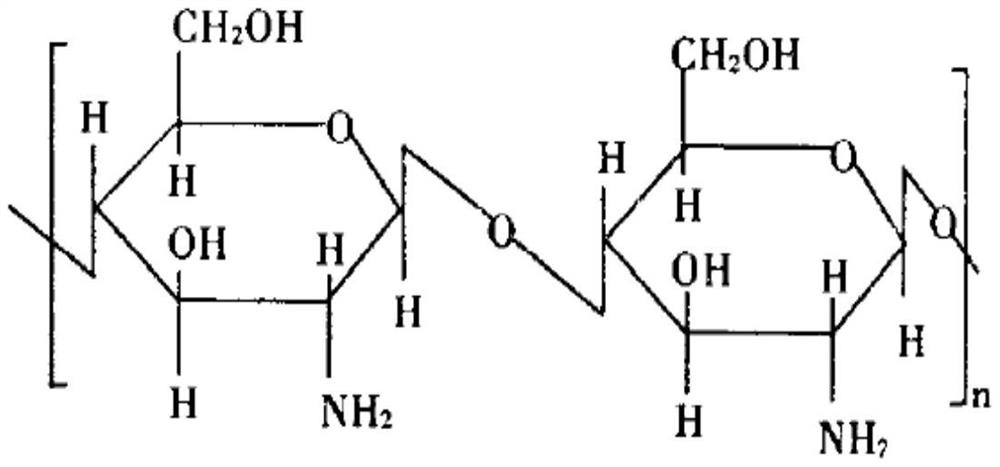
[0075] Carboxymethyl chitosan 1.4,
[0076] PVA (EG-40) 0.6,
[0077] Compound toughener 1.5 (including PEG-2000 1, glycerol 0.5),
[0078] Polyhexamethyleneguanidine 0.5,
[0079] ferric chloride 1.1.
[0080] The preparation method of the present embodiment comprises the following steps in turn:
[0081] In the aqueous solution 100mL that contains the carboxymethyl chitosan of 1.4g and 0.6gPVA (EG-40), add the composite toughener of 1.5g and the polyhexamethylene guanidine of 0.5g, mix under room temperature condition, After stirring and foaming for 30 minutes, add 10 mL of ferric chloride solution with a concentration of 10% by mass, then put it into a mold, pre-freeze at -20°C and thaw at room temperature, repeat 5 times. Materials at -20°C were demoulded and immediately freeze-dried for 24 hours. Th...
Embodiment 2
[0083] The hemostatic material of this embodiment is prepared by the first method, and the hemostatic material includes the following raw materials in parts by weight:
[0084] Carboxymethyl chitosan 1.2,
[0085] PVA (EG-40) 0.8,
[0086] Compound toughener 1.5 (including PEG-2000 1, glycerin 0.5)
[0087] Polyhexamethyleneguanidine 0.5,
[0088] ferric chloride 1.1.
[0089] The preparation method of the present embodiment comprises the following steps in turn:
[0090] In the aqueous solution 100mL that contains the carboxymethyl chitosan of 1.2g and 0.8gPVA (EG-40), add the composite toughener of 1.5g and the polyhexamethylene guanidine of 0.5g, mix under room temperature condition, After stirring and foaming for 30 minutes, add 10 mL of ferric chloride solution with a concentration of 10% by mass, then put it into a mold, pre-freeze at -20°C and thaw at room temperature, repeat 5 times. Materials at -20°C were demoulded and immediately freeze-dried for 24 hours. The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| voidage | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com