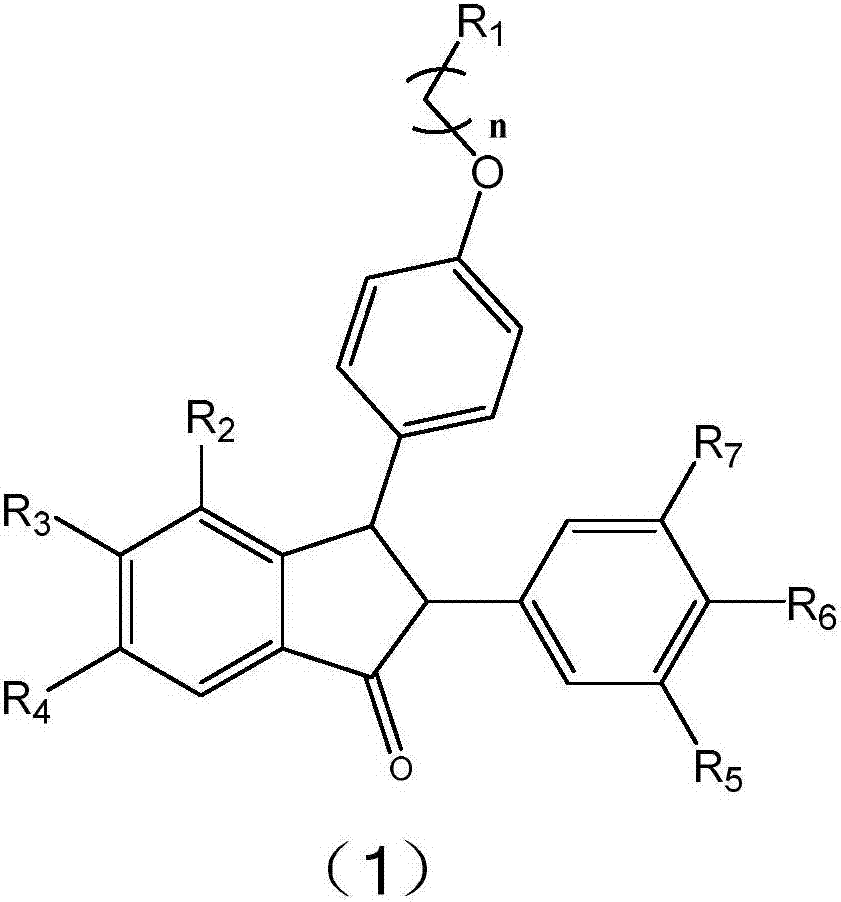
Selective ER-beta receptor regulator and medicinal application thereof
A β-receptor and selective technology, which is applied in drug combinations, antineoplastic drugs, active ingredients of heterocyclic compounds, etc., can solve problems such as cross-resistance, poor selectivity, systemic side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
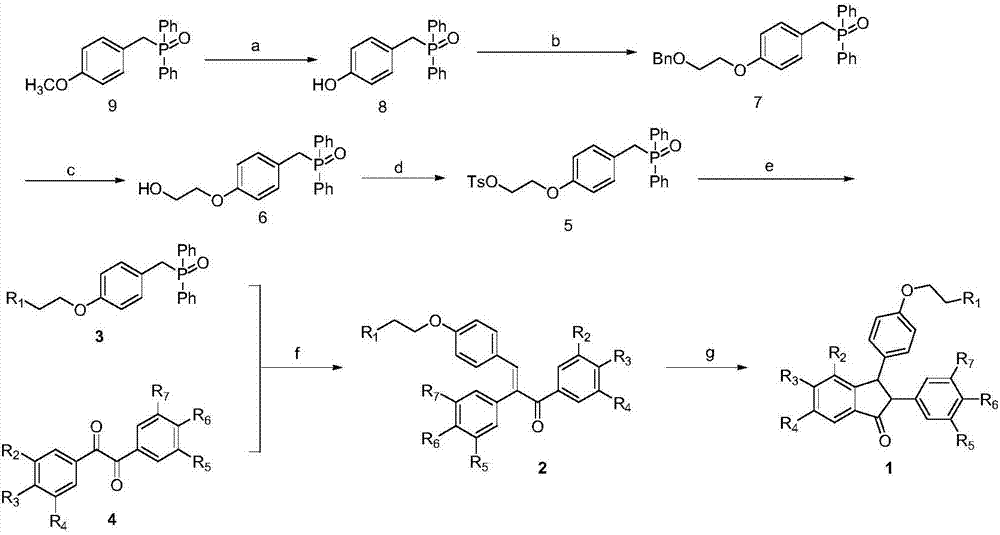
[0019] Embodiment 1: Synthesis of amine alkyl side chain class isopaucifloral F derivatives
[0020]
[0021] Using 4-methoxyphenylmethylene diphenylphosphine oxide as the starting material to synthesize compound 2 through multi-step reactions (refer to the method explored by Sun Xun, Zhong Chen, etc.);
[0022] Compounds 1a-f, 1i-m: Weigh 0.2 mmol of compound 2 and dissolve in 15 mL of anhydrous dichloromethane under nitrogen protection. Add 3 mmol of boron tribromide ether solution under ice-cooling, slowly rise to room temperature and stir for 8 h, add a small amount of water to terminate the reaction. Dichloromethane was spin off, and saturated NaHCO 3 Aqueous solution to make the solution weakly alkaline, EA extraction, water and saturated NaCl solution washing, anhydrous NaCl 2 SO 4 dry. After concentrating the solvent, it was purified by reverse phase silica gel column chromatography (CH3CN:H2O=1:2,+0.1%TFA) to obtain light brown bubbly solids 1a-f, 1i-m;
[002...
Embodiment 2
[0061] Proliferation inhibitory effect of aminoalkyl side chain isopaucifloral F derivatives on human breast cancer cell lines MCF-7, MDA-MB-231 and human non-small cell lung cancer cell line A549
[0062] Experimental method: The in vitro antitumor activity of the complexes against MCF-7, MDA-MB-231 and A549 was determined by MTT method, and tamoxifen, raloxifene and isopaucifloral F were used as positive controls. Observe the inhibition of tumor cell growth by the drug at different concentrations, and calculate its half inhibition rate (IC50 value) to evaluate its anti-tumor activity in vitro;
[0063] Take cells in logarithmic growth phase and in good condition, add an appropriate amount of trypsin (purchased from GIBCO) to digest the cells, then remove the trypsin, wash with serum-containing culture medium to collect and resuspend the cells, count, and adjust the cell density. The cell suspension was inoculated on a 96-well plate (100 μL, 4000-5000 cells / well), at 37°C, 5%...
Embodiment 3
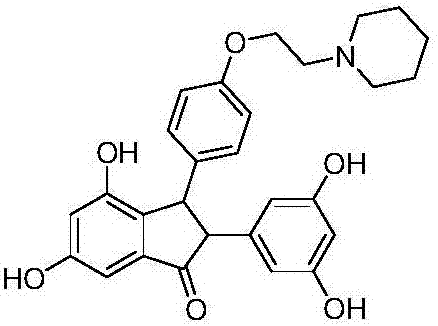
[0067] Example 3: Determination of ERβ binding ability and selectivity of compound (-)1n
[0068] This example adopts the radioligand binding assay experiment, radioligand-binding assay (radioligand-binding assay, RBA) is a classic method for testing the binding ability of ligands and receptors, which mainly uses The specific binding between the receptor and the receptor is used to study the affinity of the receptor, the number of the receptor and the subtype of the receptor. Usually, as long as a certain amount of radiolabeled ligand and the compound to be tested are incubated with the receptor to form a receptor-ligand complex, the complex is removed and then the amount of free radioactive ligand is measured, the compound and the compound can be deduced. Receptor affinity. In the current research on estrogen receptor ligands, the selectivity of compounds to a single receptor is mainly measured by this method.
[0069] In this example, the maximum binding ability of compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com