Docetaxel target prodrug for preventing liver cancer and pharmaceutical applications thereof
An anti-hepatic cancer, taxane technology, applied in the field of biomedicine, can solve the problems of poor selectivity and high systemic toxicity of docetaxel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
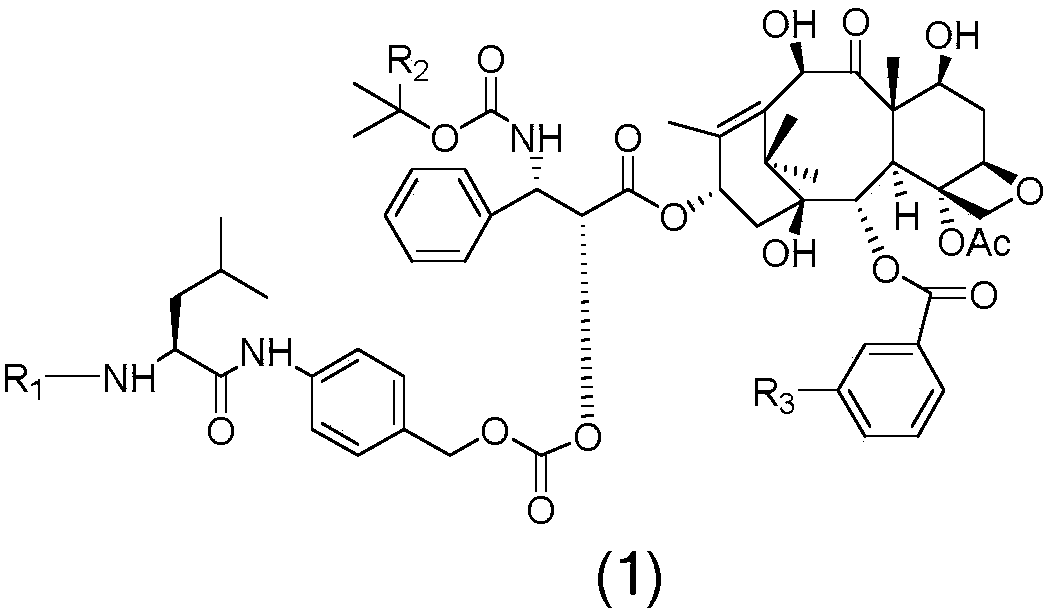
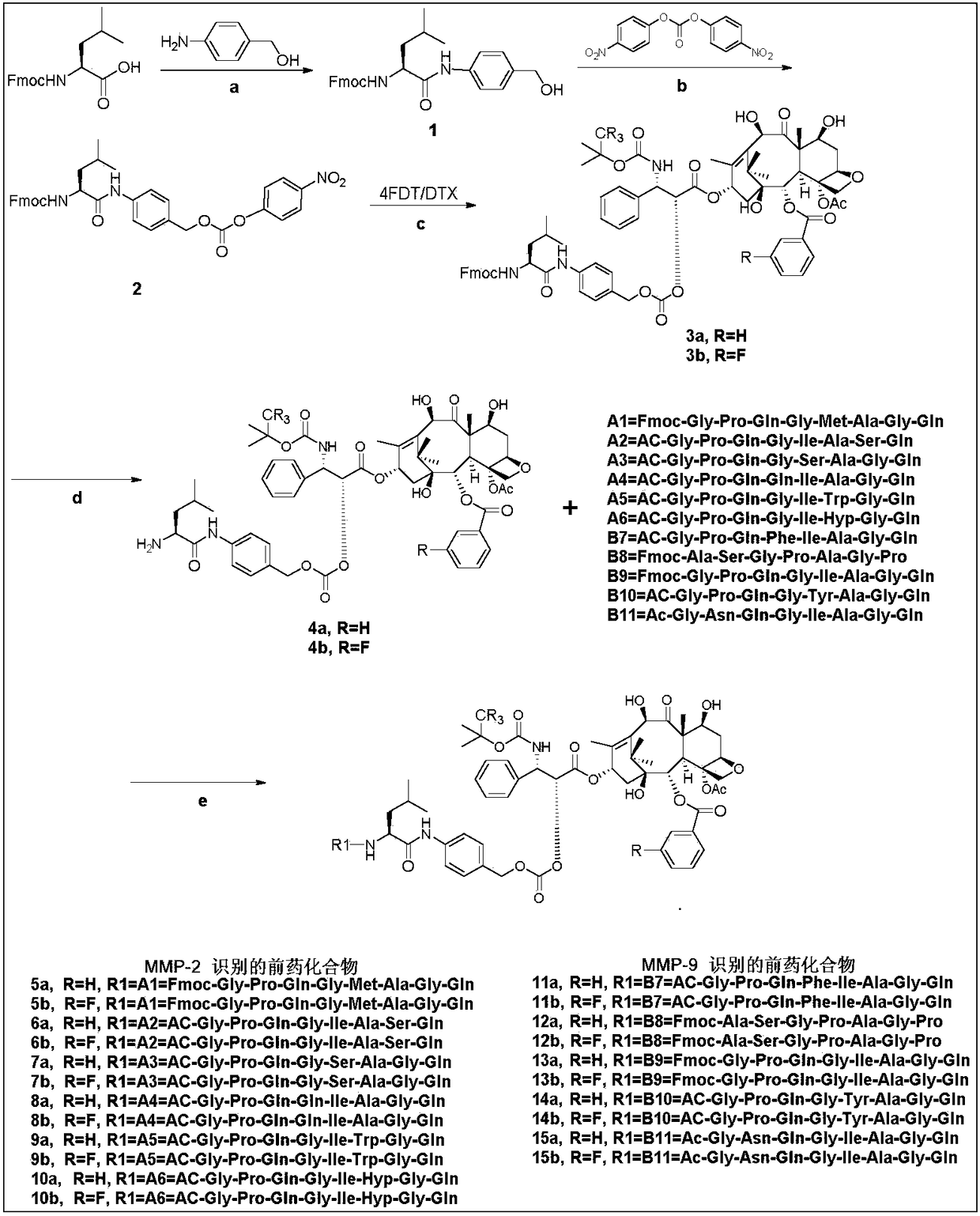
[0021] Example 1 Preparation of prodrugs coupled with docetaxel or tetrafluorodcetaxel via bridging group Leu-PABOH and liver cancer targeting polypeptide A1-B11
[0022] Taxane targeting prodrugs are prepared by coupling paclitaxel (DTX) or tetrafluorodcetaxel (4FDT) with liver cancer targeting polypeptides A1-B11, and the synthesis route includes the following steps:
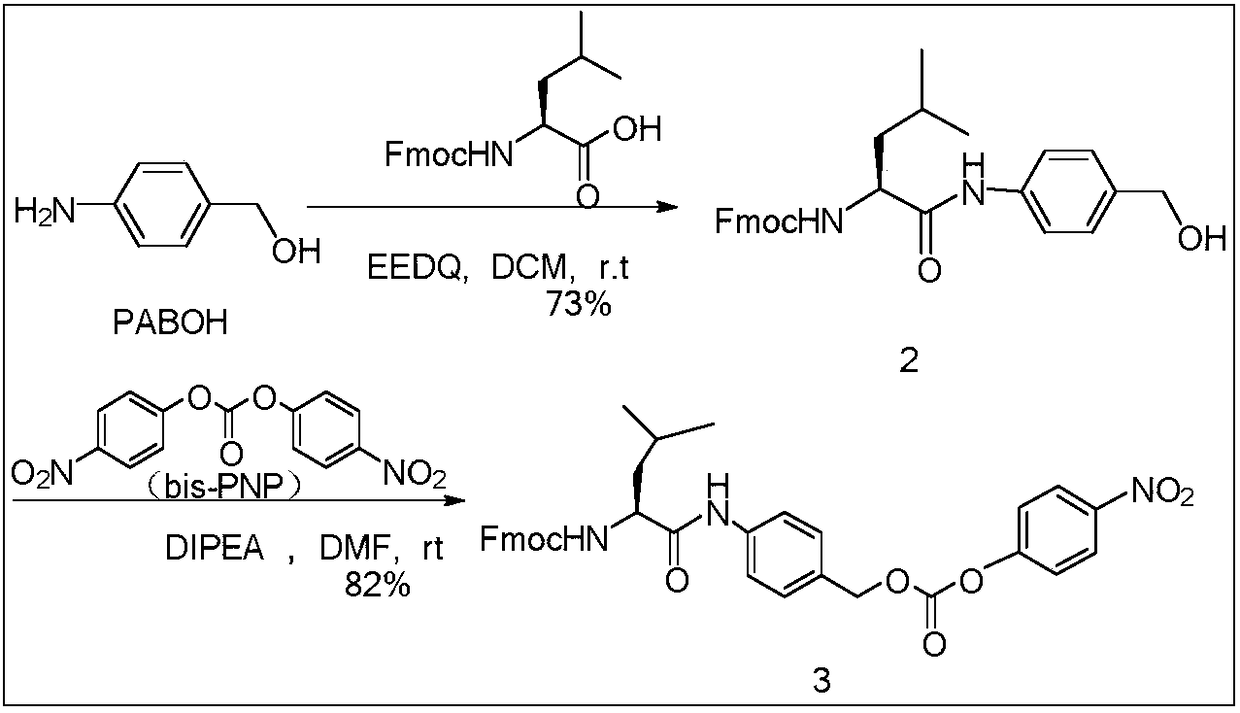
[0023] 1) Synthesis and activation modification of bridging group Leu-PABOH
[0024]
[0025]Take a 100mL round bottom flask, add 800mg (2.27mmol) of Fmoc-L-leucine, add 300mg (2.5mmol) of aminobenzyl alcohol, add 50mL of anhydrous DCM, and stir at room temperature for 10min. Then, 610 mg (2.5 mmol) of 2-ethoxy-1-ethoxycarbonyl-1,2-dihydroquinoline was added, and the reaction was stirred at room temperature. After 24 hours, TLC plate (dichloromethane:methanol=35:1) monitored the reaction. After the raw material Fmoc-L-leucine disappeared, water was added to stop the reaction, washed with saturated sodium c...
Embodiment 2
[0126] Example 2: (Tetrafluoro) docetaxel anti-hepatoma targeted prodrug has in vitro proliferation inhibitory activity on human liver cancer cell lines HepG2 and SMMC-7721 and toxicity to normal liver cell line HL7702 and normal kidney cell line HEK293
[0127] Experimental method: MTT method was used for in vitro cell assay. Sorafenib and 10-hydroxycamptothecin were used as positive controls, and docetaxel and tetrafluorodcetaxel were used as parent drug controls to observe the inhibition of the drug on cell growth at different concentrations, and calculate its half inhibitory rate ( IC 50 value) to evaluate its anti-hepatic tumor activity and toxicity to normal cells in vitro.
[0128] HepG2 cells and HEK293 cells were cultured in DMEM medium containing 10% fetal bovine serum, and SMMC-7721 and HL-7702 cells were cultured in 1640 medium containing 10% fetal bovine serum. When the cells are in the dividing phase, add trypsin to digest and collect the cells, and adjust the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com