Micro-molecule modified taxane water soluble prodrug and pharmaceutical applications thereof
A taxane, water-soluble technology, applied in the field of biomedicine, can solve the problem of easily causing allergic reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
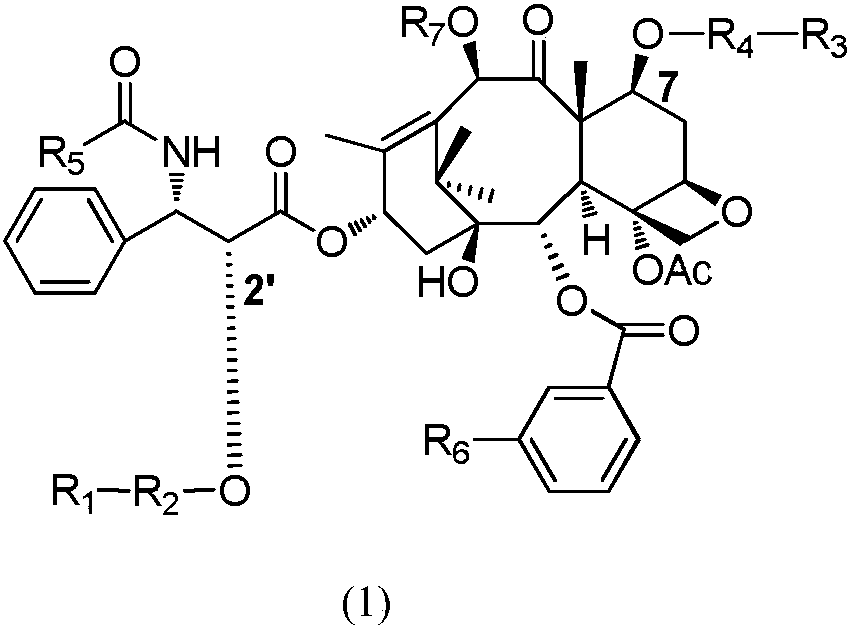
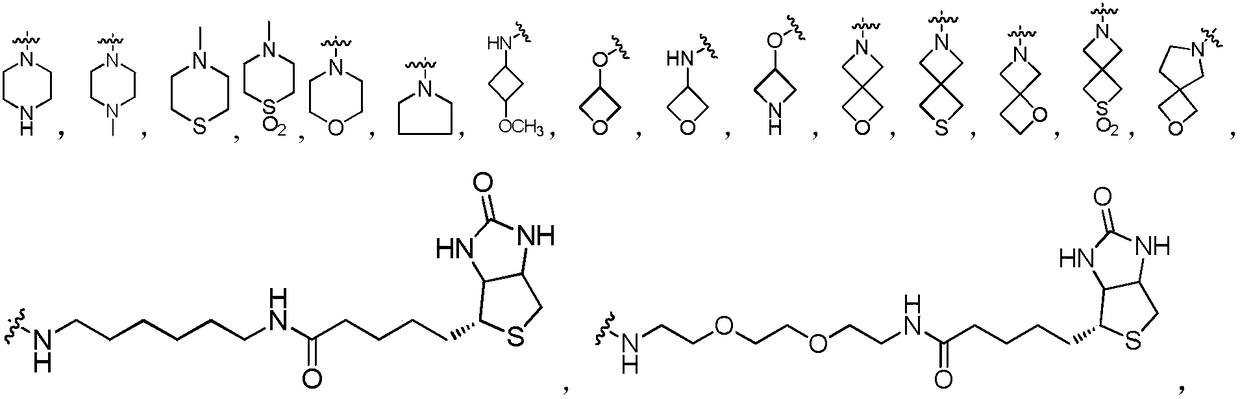
[0034] Example 1: Synthesis of 2'-O-[succinyl]-N-de-tert-butoxycarbonyl docetaxel (6).
[0035]
[0036] Take a 100 mL round bottom flask, add 200 mg (0.25 mmol) of docetaxel, then add 375 mg (3.75 mmol) of succinic anhydride, add 10 mL of anhydrous pyridine, and stir the reaction at room temperature. After 24 hours, monitor the reaction on a TLC plate (take a small amount of the reaction solution, Add saturated sodium bicarbonate solution and ethyl acetate, take the ethyl acetate layer and dot the plate), the raw material point basically disappeared, stop the reaction, evaporate the pyridine to dryness, then pump the oil for 30 minutes, after the pyridine is almost completely removed, add 30 mL ethyl acetate , Washed with saturated sodium bicarbonate (20mL), washed with saturated sodium chloride solution (20mL), dried the organic phase with anhydrous sodium sulfate, filtered and spin-dried the solvent to obtain the crude product purified by silica gel column chromatography (dichl...
Embodiment 2
[0037] Example 2: Synthesis of 2'-O-[succinyl]paclitaxel (7). The operation is the same as the synthesis of 6.
[0038]
[0039] The yield was 75%. 1 H NMR(400MHz, acetone-d 6 ):δ ppm 1.18(s,3H,17-CH 3 ), 1.19(s, 3H, 16-CH 3 ),1.66(s,3H,19-CH 3 ),1.94(s,3H,18-CH 3 ),1.78and 2.32(m,2H,6-CH 2 ), 2.16(s,3H,10-OAc), 2.20(m,2H,14-CH 2 ),2.46(s,3H,4-OAc),2.62and 2.69(m,4H,2’-COCH 2 CH 2 COOH), 3.83(d,1H,J=7.2Hz,3-CH),4.06(m,1H,7-OH),4.16(m,2H,20-CH 2 ), 4.42(m,1H,7-CH), 4.96(d,1H,J=9.6Hz,5-CH),5.55(d,1H,J=6Hz,2'-CH),5.67(d,1H ,J=7.2Hz,2-CH),5.96(m,1H,3'-CH),6.15(t,1H,J=8.4Hz,13-CH),6.42(s,1H,13-CH), 7.30(t,1H,J=7.6Hz,3'-CONH),7.46(m,4H,o,m-Ph),7.53(t,1H,J=7.2Hz,p-Ph),7.61(m, 4H, o, m-Ph), 7.69 (t, 1H, J = 7.2 Hz, p-Ph), 7.87 (d, 2H, J = 7.2 Hz, m-OBz), 8.13 (d, 2H, J = 7.2 Hz,o-OBz),8.48(d,1H,J=9.2Hz,p-OBz).ESI-MS:m / z 954.4[M+H] + ,976.3[M+Na] + .C 51 H 55 NO 17 ..
Embodiment 3
[0040] Example 3: 2'-O-[succinyl]-N-de-tert-butoxycarbonyl-N-[2-(1,1,1-trifluoro-2-methyl)-propoxycarbonyl]-2 -Synthesis of debenzoyl-2-m-fluorobenzoyl docetaxel (8). The operation is the same as the synthesis of 6.
[0041]
[0042] The yield was 68%. 1 H NMR(400MHz,acetone-d 6 ):δ ppm 1.13(s,3H,17-CH 3 ),1.17(s,3H,16-CH 3 ),1.59and 1.61(s,6H,3’-(CH 3 ) 2 ),1.71(s,3H,19-CH 3 ),1.89(s,3H,18-CH 3 ),1.97and2.30(m,2H,6-CH 2 ),2.21(m,2H,14-CH 2 ),2.44(s,3H,4-OAc),2.64and 2.69(m,4H,2’-COCH 2 CH 2 COOH),3.90(d,1H,J=7.2Hz,3-CH),4.17(s,2H,20-CH 2 ), 4.30(m,1H,7-CH), 4.96(d,1H,J=9.2Hz,5-CH),5.22(s,1H,10-CH),5.36-5.39(m,2H,2' and 3'-CH), 5.64(d,1H,J=7.6Hz,2-CH), 6.10(t,1H,J=8.4Hz,13-CH), 7.29(t,1H,J=6.4Hz, 3'-CONH-),7.45-7.53(m,5H,3'-Ph),7.66(m,2H,OBz),7.78(d,1H,J=9.2Hz,OBz),7.94(d,1H, J=8.4Hz,OBz); ESI-MS: m / z 980.3[M+H] + ,997.5[M+H 2 O] + .C 47 H 53 F 4 NO 17 ..
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com