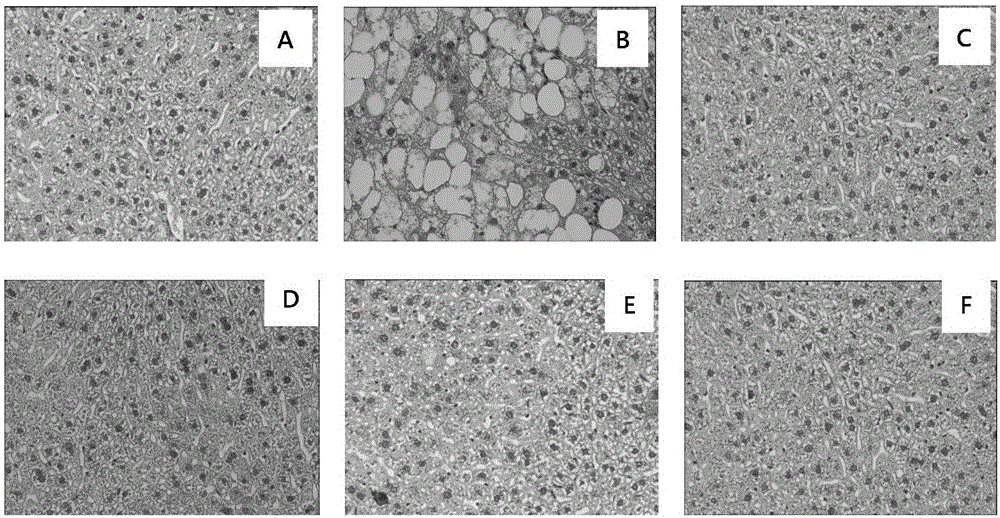
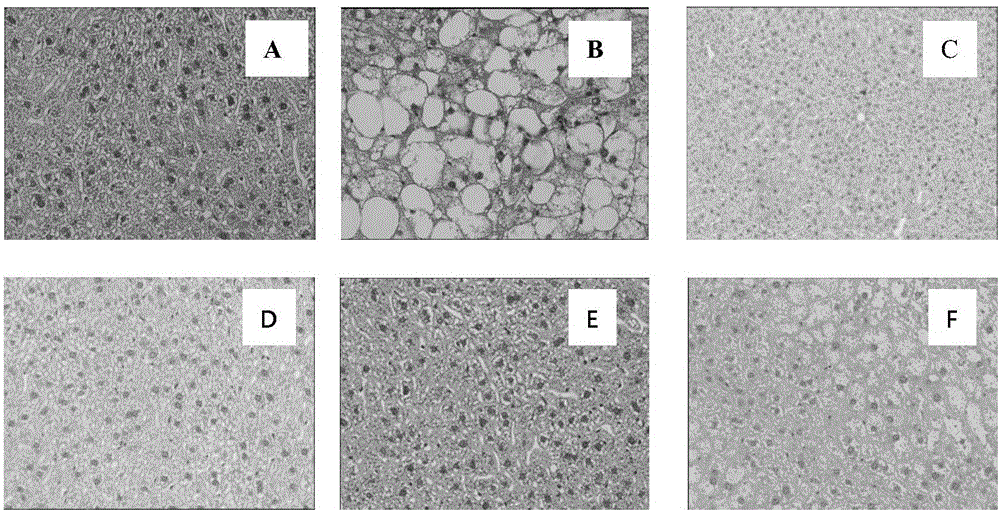
Application of p-dihydroxybenzene farnesyl compounds
A technology of hydroquinone farnesyl compounds, which is applied in the application field of hydroquinone farnesyl compounds, can solve the problems of curative effect failure, anti-tumor chemotherapy curative effect reduction, etc., and achieve reduction in treatment and prevention, cancer prevention or inhibition The effect of tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The preparation of compound shown in embodiment 1, formula 1-17
[0073] (1) Prepare the extract of Ganoderma lucidum
[0074] Ganoderma lucidum fruiting body is cut into pieces, weighing 5000 grams. Reflux extraction with 15L aqueous solution of 95% ethanol by volume for 3 times, 1 hour each time. The extracts were combined, concentrated and dried under reduced pressure to obtain 170 g of extract.
[0075] The extract was further dissolved with 600 ml of distilled water, extracted three times with an equal volume of n-hexane, and the organic phase was discarded. The aqueous phase was extracted three times with an equal volume of ethyl acetate, the aqueous phase was discarded, and the ethyl acetate extracts were combined. Evaporate ethyl acetate to dryness with a rotary evaporator (RE-52AA, purchased from Shanghai Yarong Biochemical Instrument Factory) to obtain 54 g of extract, which is designated as LZ-E.
[0076] (2) Preparation of compounds
[0077] The LZ-E pr...
Embodiment 2
[0085] The synthesis of compound shown in embodiment 2, formula 3 and formula 4
[0086] For the synthetic route, please refer to literature Yajima, A.; Urao, S.; Katsuta, R.; Nukada, T. Eur. J. Org. Chem.2014, 731-738.
[0087]
[0088] 2,5-bis(methoxymethoxy)benzaldehyde (1): 2,5-dihydroxybenzaldehyde (400mg, 2.90mmol) cooled to 0°C, potassium carbonate (4.09g, 29.0mmol) Chloromethyl methyl ether (689uL, 8.70mmol) was added to the acetone solution (60ml), and stirred at room temperature for 10 hours. Diluted with water (60ml) followed by extraction three times with ethyl acetate (60ml), the combined organic layers were extracted with water and brine, dried over sodium sulfate. After vacuum concentration, the extract was purified by silica gel column chromatography (n-hexane / ethyl acetate, 3:1) to obtain a colorless oily substance 2,5-bis(methoxymethoxy)benzaldehyde (1) (642mg, 98% ).
[0089] (E)-3-[2,5-bis(methoxymethoxy)benzene]propenyl acetate (2): to anhydrous solu...
Embodiment 3
[0108] Confirmation of the structure of the compound shown in embodiment 3, formula 1-17
[0109] The 17 prepared compounds were detected by nuclear magnetic resonance, infrared and mass spectrometry to determine the structure of each compound.
[0110] The nuclear magnetic resonance instrument used is BrukerMercury-500 and BrukerMercury-600 megahertz (Bruker Spectrum Instrument Company), the infrared chromatograph is Nicolet IS5FT-IR (U.S. Nicolet Instrument Co., Ltd.), and the mass spectrometer is Bruker APEX III 7.0T and APEX II FT-ICR (Bruker Spectroscopy Instruments).
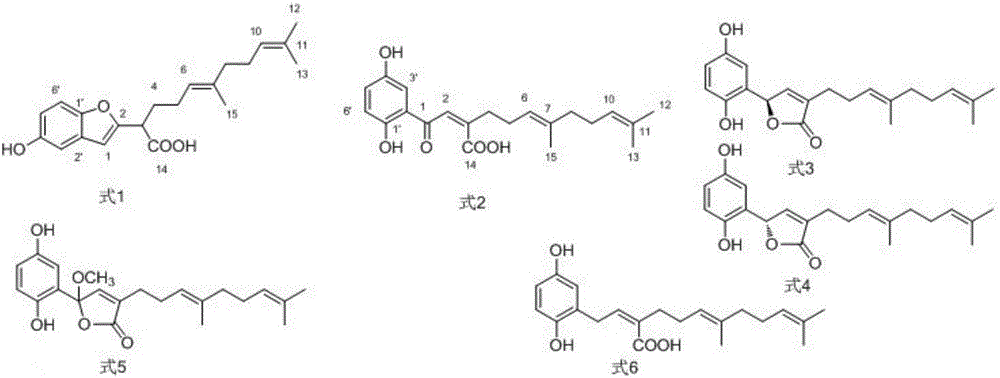
[0111] It was confirmed that these 17 compounds are all known compounds and have a common hydroquinone farnesyl structure.
[0112] Tables 1 to 8 show the NMR carbon spectrum and hydrogen spectrum confirmation data of each compound.
[0113] Compound 1: [(E)-2-(5-Hydroxybenzofuran-2-yl)-6,10-dimethyltetradecane-5,9-geranic acid](E)-2-(5 -hydroxybenzofuran-2-yl)-6,10-dimethylundeca-5,9-dienoic acid
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com