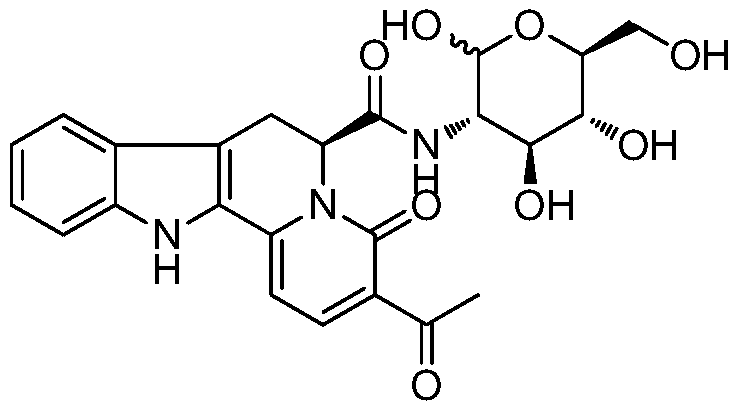
Indoloquinazine-6-formyl-3-glucosamine, its preparation, activity and application
A technology of glucosamine and quinozine, which is applied in the field of indoloquinazine-6-formyl-3-glucosamine, its preparation, activity and application, and can solve the problems of worsening prognosis of tumor patients and poor prognosis of tumor patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
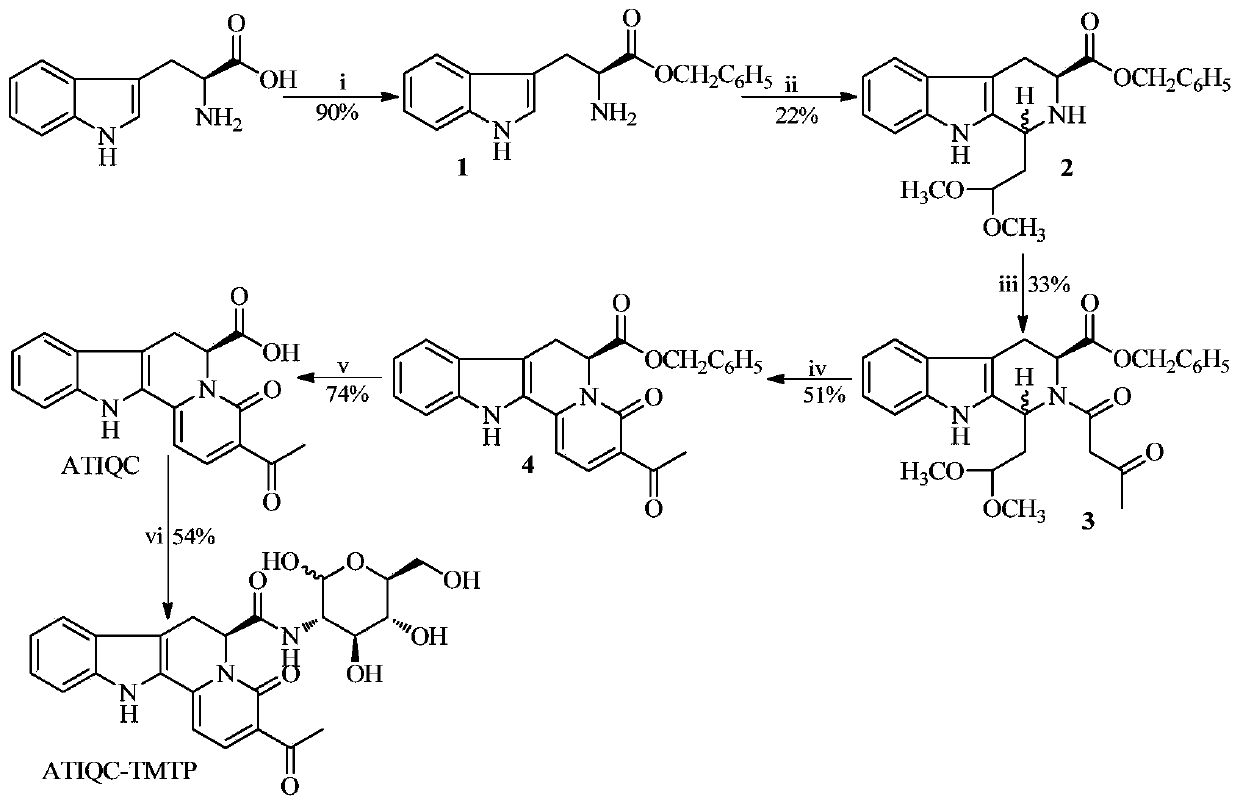
[0019] Embodiment 1 prepares L-tryptophan benzyl ester (1)
[0020] 20.40 g (100.0 mmol) of L-tryptophan, 13.00 g (120 mmol) of benzyl alcohol and 40.60 g (120.0 mmol) of polyphosphoric acid were reacted at 80° C. for 72 hours. The reaction solution was lowered to room temperature, 30 mL of anhydrous ether was added, and stirred for 2 hours to precipitate a colorless solid, which was filtered. The filter cake was washed repeatedly with ether to obtain 35.40 g (90%) of the title compound as a colorless solid.
Embodiment 2
[0021] Example 2 Preparation of (3S)-1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (2)
[0022] Add 8mL (48.8mmol) 1,1,3,3-tetramethoxypropane and 9mL trifluoroacetic acid to 123mL dichloromethane successively under stirring in an ice bath, and activate for 40 minutes. Then add 10.00g (25.5mmol) L-tryptophan benzyl phosphate, stir at room temperature for 120 hours, the reaction is over. Slowly add saturated Na with stirring in an ice bath 2 CO 3 aqueous solution, stirred vigorously until the pH of the aqueous layer was 7, and the separated dichloromethane layer was sequentially washed with 5% NaHCO 3 aqueous solution and saturated NaCl aqueous solution. Dichloromethane layer with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated to dryness under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether: acetone = 3:1) to obtain 2.21 g (22%) of the title compound as...
Embodiment 3
[0023] Example 3 Preparation of (3S)-1-(2,2-dimethoxyethyl)-2-acetoacetyl-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (3)
[0024] Dissolve 2.93g (7.4mmol) (3S)-1-(2,2-dimethoxyethyl)-2,3,4,9-tetrahydro-β-carboline-3-carboxylic acid benzyl ester in 50mL Acetone, add 0.9mL diketene and 0.6mL triethylamine under stirring in an ice bath, stir at room temperature for 17 hours, TLC shows that the raw materials completely disappear. Add 2.5 mL of distilled water to the reaction mixture under ice-bath stirring, stir for 1 hour, concentrate under reduced pressure to remove acetone, and extract the residue three times with dichloromethane. The combined dichloromethane layers are sequentially washed with 5% NaHCO 3 aqueous solution and saturated NaCl aqueous solution, and the dichloromethane layer was washed with anhydrous NaCl 2 SO 4 After drying and filtering, the filtrate was concentrated to dryness under reduced pressure and purified by silica gel column chromat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com