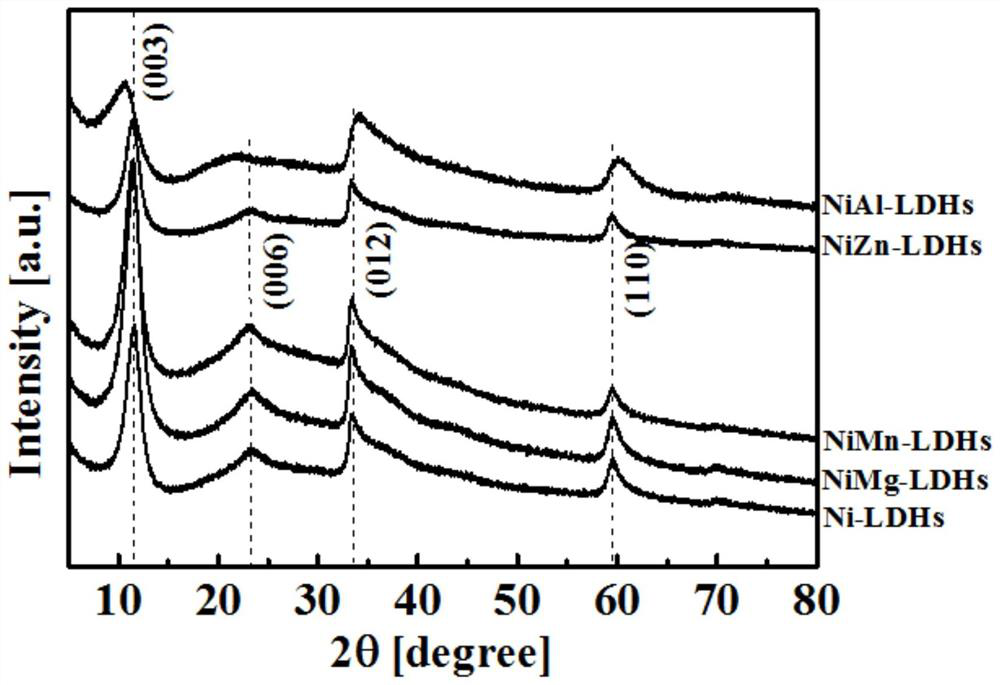
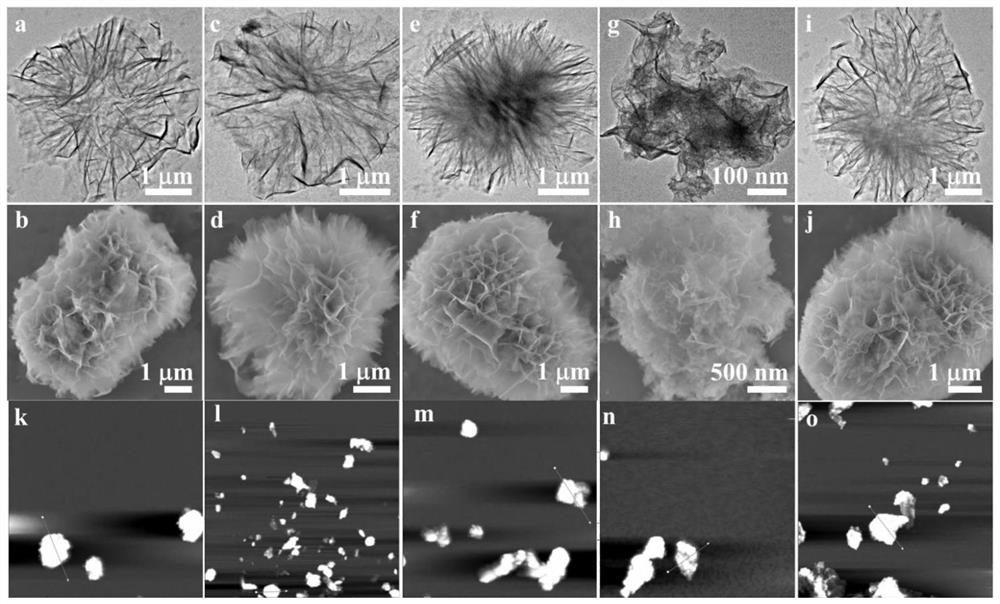
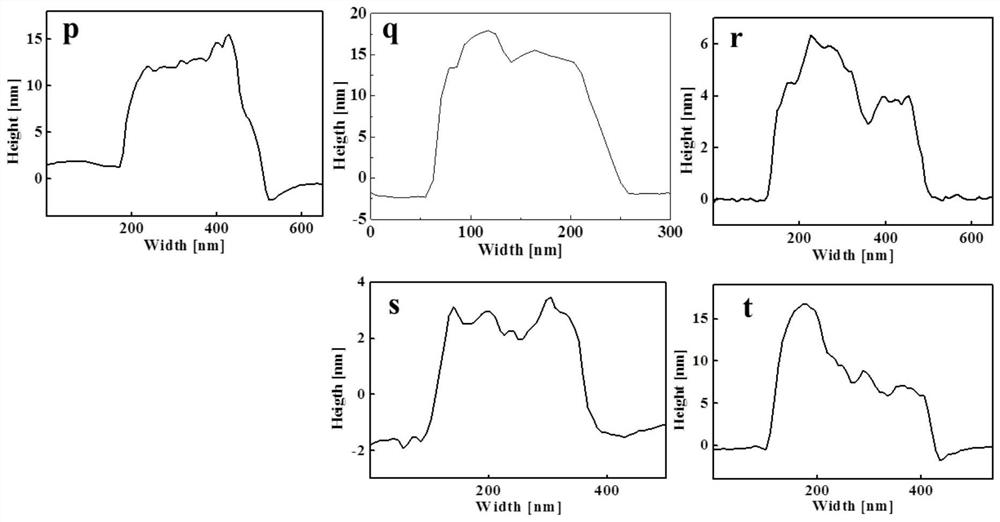
A preparation method of doping different metal ions to regulate nickel-based double metal hydroxide
A technology of hydroxide and metal ions, applied in the direction of metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of limited application, expensive, scarce elements, etc. to improve performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Weigh 5 mmol of nickel nitrate, 0.1 mmol of magnesium nitrate and 5 mmol of 2-methylimidazole in a 20 ml polytetrafluoroethylene kettle.
[0018] (2) Add a certain amount of anhydrous methanol solution into the kettle so that the concentration of magnesium nitrate is 1.71g / L, ultrasonically or stir until completely dissolved.
[0019] (3) Place the polytetrafluoroethylene kettle in a stainless steel reaction kettle, and tighten the kettle cap.
[0020] (4) The stainless steel reaction kettle was placed in an oven and set to react at 80°C for 4 hours, the reaction was stopped, and the temperature was lowered to room temperature.
[0021] (5) Centrifuge at a speed of 10000-12000rpm in a centrifuge for 3-5min to collect the precipitate in the reaction solution, wash the precipitate repeatedly with methanol solution until the supernatant is colorless and transparent, then put the precipitate in a vacuum drying oven at 60°C for 8 hours, Finally, Ni-Mg LDHs powder was ob...
Embodiment 2
[0023] (1) Weigh 5 mmol of nickel nitrate, 0.1 mmol of manganese nitrate and 5 mmol of 2-methylimidazole in a 20 ml polytetrafluoroethylene kettle.
[0024] (2) Add a certain amount of anhydrous methanol solution into the kettle so that the concentration of manganese nitrate is 1.67g / L, sonicate or stir until completely dissolved.
[0025] (3) Place the polytetrafluoroethylene kettle in a stainless steel reaction kettle, and tighten the kettle cap.
[0026] (4) The stainless steel reaction kettle was placed in an oven and set to react at 80°C for 4 hours, the reaction was stopped, and the temperature was lowered to room temperature.
[0027] (5) Centrifuge at the speed of 8000-9000rpm in the centrifuge for 8-10min to collect the precipitate in the reaction solution, wash the precipitate repeatedly with methanol solution until the supernatant is colorless and transparent, then put the precipitate in a vacuum drying oven at 50°C for 10 hours, Finally, Ni-Mn LDHs powder was obta...
Embodiment 3
[0029] (1) Weigh 5 mmol of nickel nitrate, 0.1 mmol of zinc nitrate and 5 mmol of 2-methylimidazole in a 20 ml polytetrafluoroethylene kettle.
[0030] (2) Add a certain amount of anhydrous methanol solution into the kettle so that the concentration of zinc nitrate is 1.98g / L, sonicate or stir until completely dissolved.
[0031] (3) Place the polytetrafluoroethylene kettle in a stainless steel reaction kettle, and tighten the kettle cap.
[0032] (4) The stainless steel reaction kettle was placed in an oven and set to react at 80°C for 4 hours, the reaction was stopped, and the temperature was lowered to room temperature.
[0033] (5) Centrifuge at a speed of 10000-12000rpm in a centrifuge for 3-5min to collect the precipitate in the reaction solution, wash the precipitate repeatedly with methanol solution until the supernatant is colorless and transparent, then put the precipitate in a vacuum drying oven at 80°C for 4 hours, Finally, Ni-Zn LDHs powder was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com