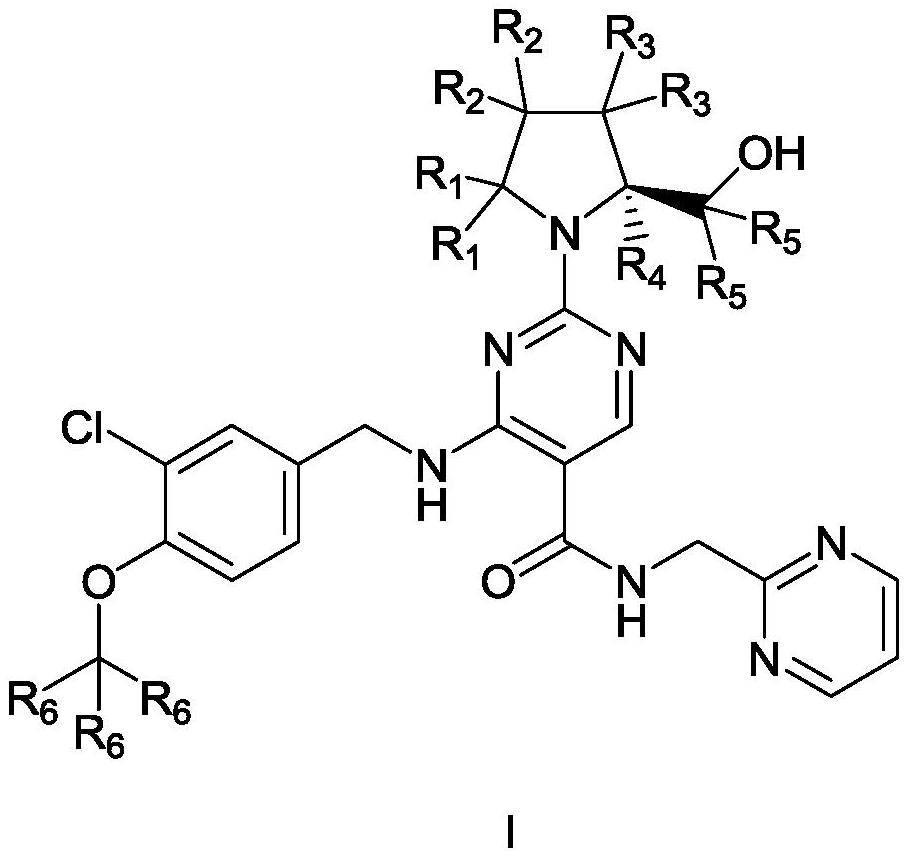
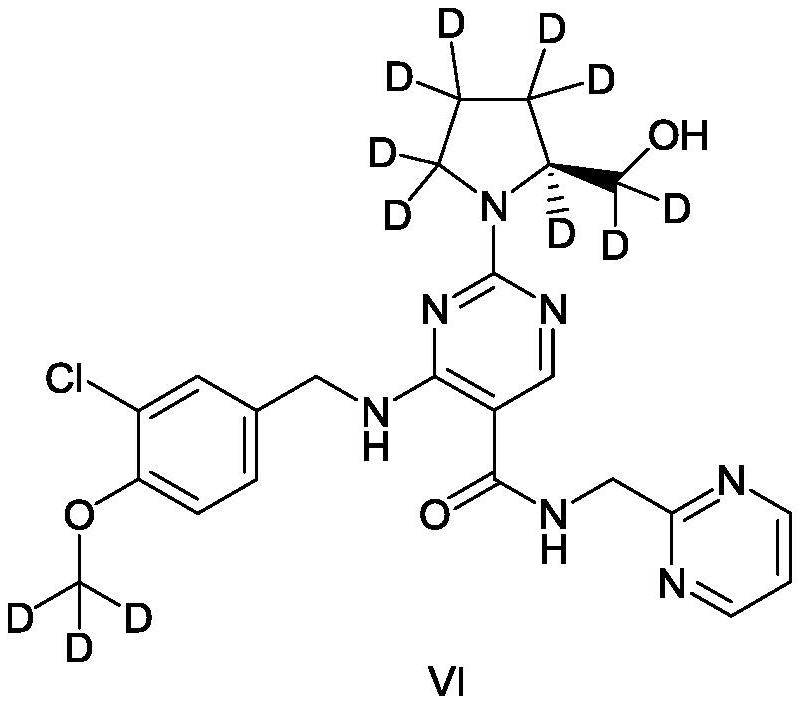
Pyrimidine carboxamide derivatives and preparation method, composition, formulation and use thereof
A technology of pyrimidine carboxamide and derivatives, applied in the field of medicine, can solve the problems of weakening smooth muscle relaxation, lowering cGMP levels, etc., and achieve the effects of avoiding side effects, reducing intake, and reducing the frequency of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation methods of solvates and hydrates are well known in the art. The typical method is as follows: Dissolve the compound in the required amount of solvent (organic solvent, water or a mixture of the two) at a temperature higher than ambient temperature In the process, the solution is cooled at a speed sufficient to form crystals, then the crystals are separated by standard methods, and finally the presence of solvent or water in the solvate or hydrate crystals is confirmed by analytical techniques (such as infrared spectroscopy, thermal analysis).
[0060] In a preferred embodiment, the above-mentioned compound represented by Formula I is a single crystal or a polymorph.
[0061] Secondly, the present invention provides a preparation method of the above-mentioned compound represented by formula I, which comprises:
[0062] Step 1): Prepare 4-bromo-2-methylthio-N-(pyrimidin-2-ylmethyl)- from 4-bromo-2-methylthio-5-pyrimidinecarbonyl chloride and 2-aminomethylpyrimidi...
Embodiment 1
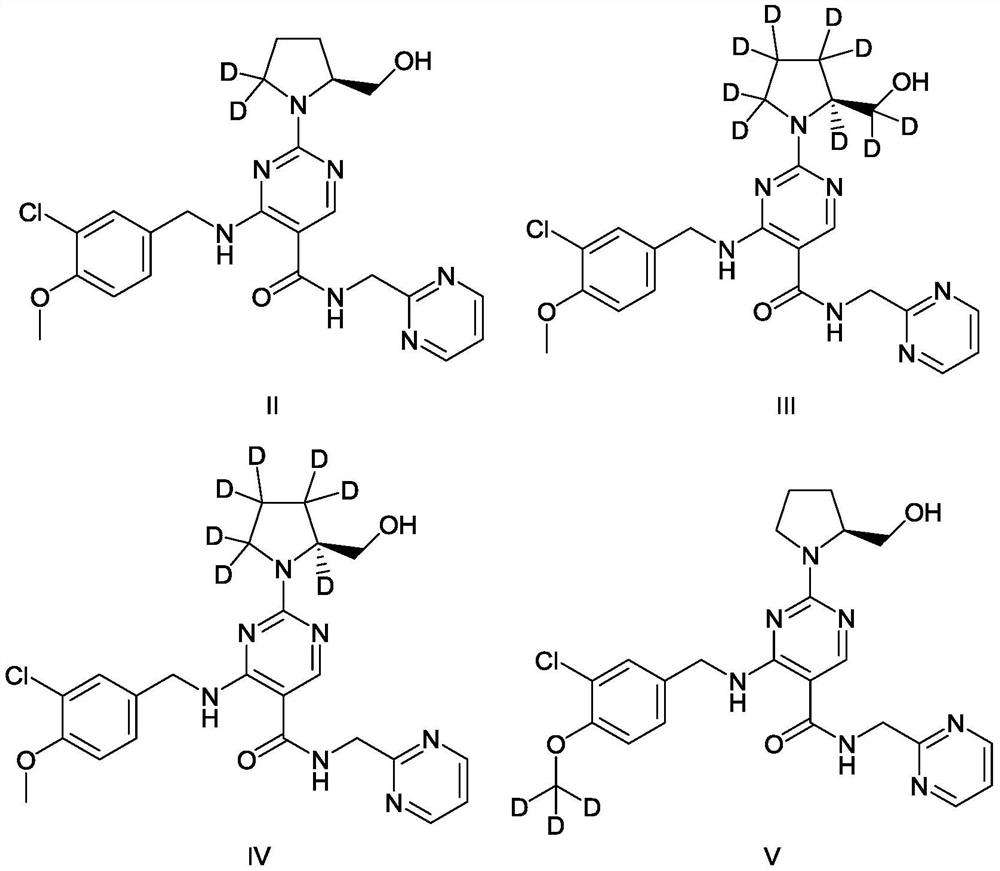
[0084] Example 1: Preparation of (S)-4-[(3-chloro-4-methoxybenzyl)amino]-2-[5,5-dideutero-2-(hydroxymethyl)pyrrolidine-1 -Yl]-N-(pyrimidin-2-ylmethyl)-5-pyrimidinecarboxamide (compound of formula II).
[0085]
[0086] Follow the procedure shown above to prepare the target compound, and the specific steps are as follows:
[0087] S1: Preparation of 4-bromo-2-methylthio-N-(pyrimidin-2-ylmethyl)-5-pyrimidinecarboxamide (compound 3):
[0088] Under the protection of argon, dissolve 4-bromo-2-methylthio-5-pyrimidinecarbonyl chloride (Compound 1) (10mmol) in anhydrous dichloromethane (20ml), and cool down in an ice bath (-10℃) , Slowly add triethylamine (20mmol), 4-dimethylaminopyridine (1mmol) and 2-aminomethylpyrimidine (compound 2) (10.5mmol) in dry dichloromethane (10ml) respectively. After the addition was complete, the mixture was heated to 25°C and stirred for 1 hour.
[0089] After LC-MS and TLC showed that the intermediate reaction was basically completed, water was added to ter...
Embodiment 2
[0102] Example 2: Preparation of (S)-4-[(3-chloro-4-methoxybenzyl)amino]-2-[2,3,3,4,4,5,5-heptadetro-2 -(2,2-Di-deuterated hydroxymethyl)pyrrolidin-1-yl]-N-(pyrimidin-2-ylmethyl)-5-pyrimidinecarboxamide (compound of formula III).
[0103] The target compound was prepared according to the procedure described in Example 1, except that 2,3,3,4,4,5,5,6,6-nonadeutero-L-prolinol (Compound 6-1 ) Replace the 5,5-dideutero-L-prolinol (Compound 6) used in S3 of Example 1 to obtain the target product. After testing, the purity of the product is greater than 97%.
[0104] LC-MS(ESI)m / z:494[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com