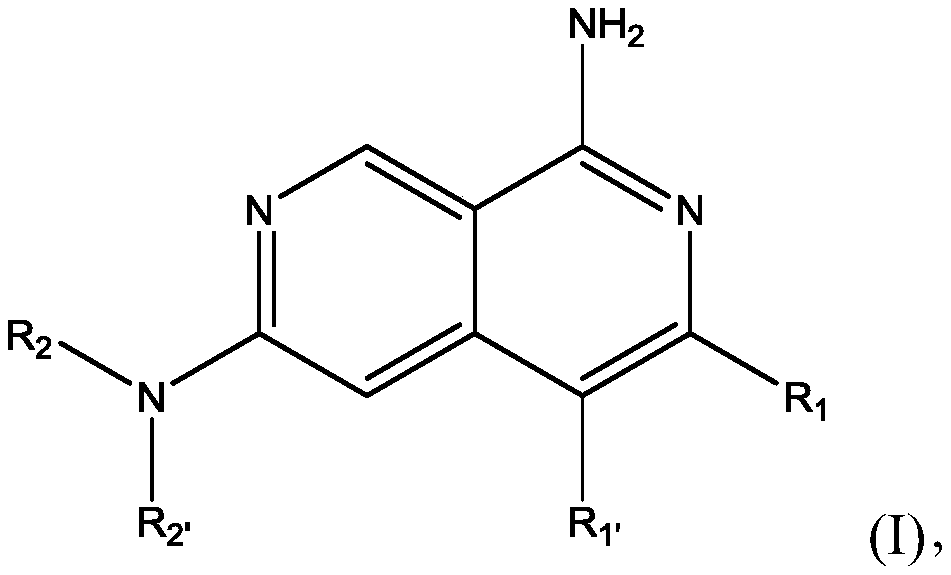
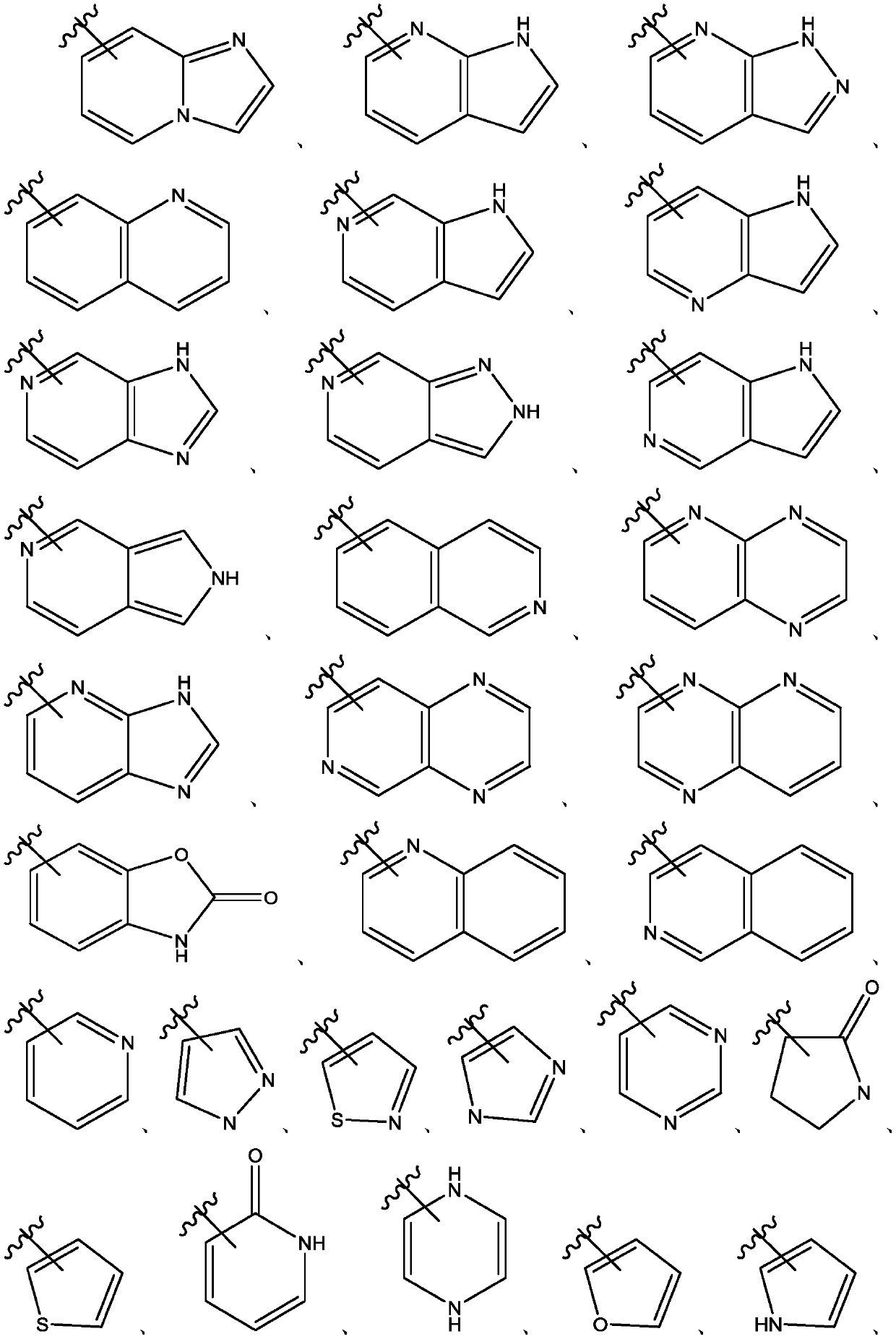
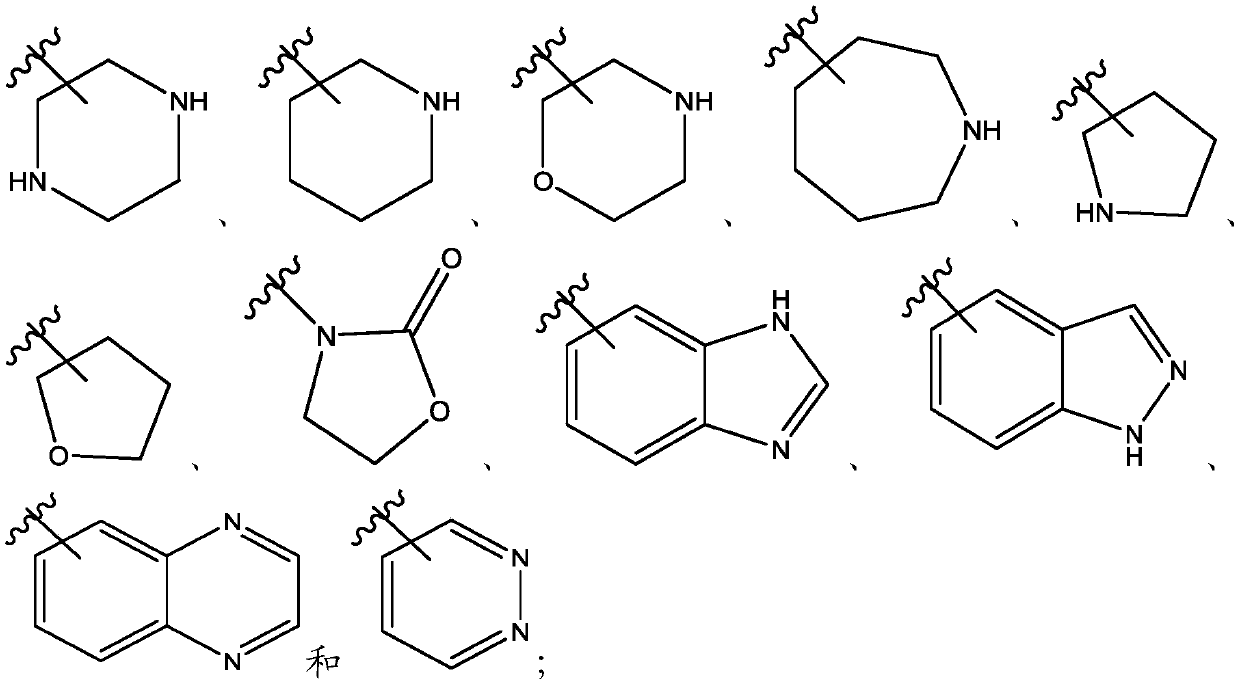
Naphthyridines as inhibitors of hpk1
A heteroaryl and heterocyclyl technology, applied in the field of compounds that regulate the function of HPK1, can solve the problems that surgical resection is not feasible and damages cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0792] Embodiment 1: synthetic intermediate
Embodiment I1
[0794] Intermediate 1: tert-butyl N-(6,8-dichloro-2,7-naphthyridine-3-yl)carbamate
[0795]
[0796] Step 1: 2,6-Dichloro-4-iodopyridine-3-carboxylic acid
[0797]
[0798] At -78°C, N 2 , to a solution of 2,6-dichloro-3-iodo-pyridine (13.69 g, 50 mmol) in anhydrous THF (150 mL) was added LDA (2.0 M in THF, 27.5 mL, 55 mmol) dropwise. After the addition was complete, the reaction solution was stirred at -78°C for 2 hours. in CO 2 After bubbling for 5 minutes, the reaction mixture was stirred at room temperature for 2 hours. The reaction solution was quenched with concentrated HCl, and extracted with dichloromethane. The combined organic layers were in Na 2 SO 4 Drying on , filtered and concentrated gave 2,6-dichloro-4-iodo-pyridine-3-carboxylic acid (8.1 g, 51% yield) as a light yellow solid. LCMS(ESI)[M+H] + = 317.8.
[0799] Step 2: (2,6-Dichloro-4-iodopyridin-3-yl)methanol
[0800]
[0801] A mixture of 2,6-dichloro-4-iodo-pyridine-3-carboxylic acid (6 ...
Embodiment I2
[0824] Intermediate 2: 6,8-dichloro-2,7-naphthyridine-3-amine
[0825]
[0826] To the vial add tert-butyl N-(6,8-dichloro-2,7-naphthyridine-3-yl)carbamate (1.05 g, 3.34 mmol), HCl / 1,4-dioxane (10 mL, 4N, 40 mmol) and dichloromethane (5 mL). The mixture was stirred at 40°C for 4 hours. The mixture was concentrated and dried in vacuo to give 6,8-dichloro-2,7-naphthyridine hydrochloride (823 mg, 96% yield) as a yellow solid. LCMS(ESI)[M+H] + = 214.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com