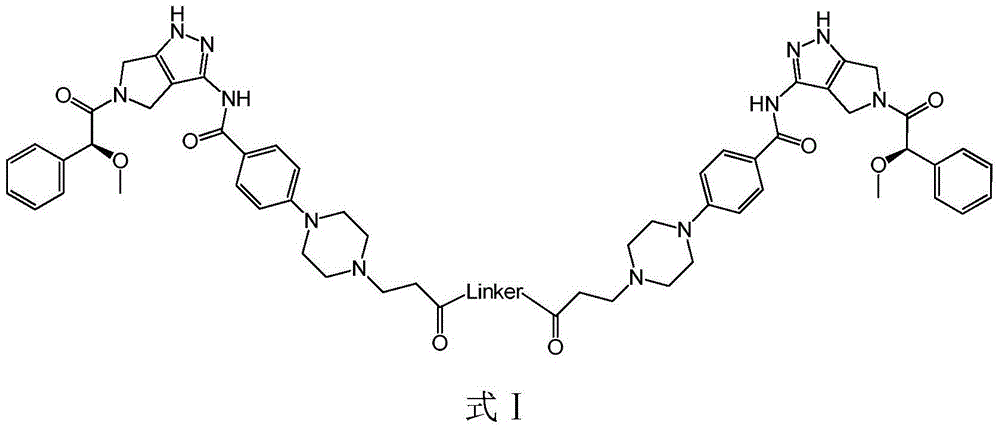
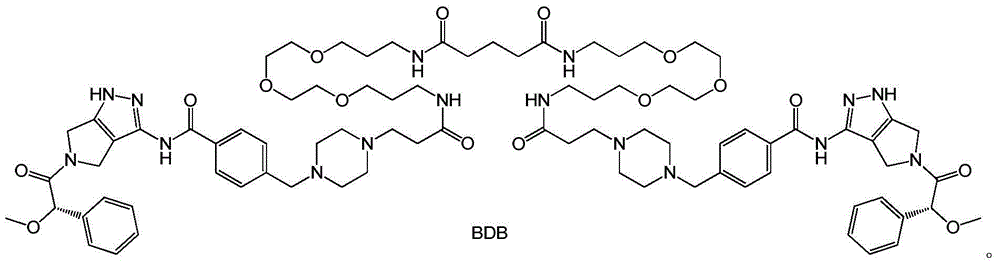
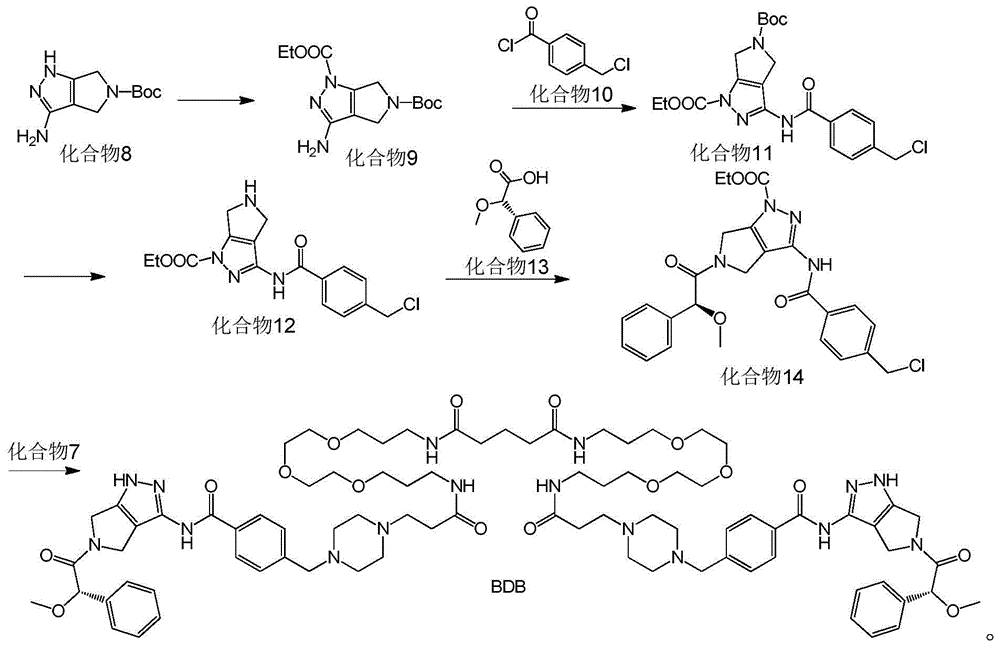
Bcr-Abl amphiploid inhibitor, preparation method and application thereof
A technology of use and molar volume, applied in a class of Bcr-Abl diploid inhibitors and the fields of preparation and use, capable of solving drug resistance, decreased affinity, and decreased affinity between imatinib and Abl kinase and other problems, to achieve the effect of inhibiting tyrosine kinase activity and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The preparation of embodiment 1 compound BDB of the present invention
[0076] The synthetic route is as follows:
[0077]
[0078]
[0079] Concrete synthetic steps are as follows:
[0080] Preparation of Compound 2:
[0081] Compound 1 (10g, 45.5mmol; manufacturer: Sigma-Aldrich) was dissolved in 500ml DCM, at room temperature, (Boc) 2 O (9.4g, 43.18mmol) was dissolved in 100ml of DCM, slowly added dropwise to the reaction bottle, and reacted overnight; 200ml of water was added to the reaction solution, and after adjusting the pH to 3-4, the organic phase impurities were extracted, and the aqueous phase Adjust the pH to 10, then add sodium chloride for saturation, extract the product, wash the organic phase with saturated sodium chloride aqueous solution (10ml×6) to remove unreacted raw materials, dry over sodium sulfate, and evaporate the solvent to obtain a colorless oil Compound 2 (4.7 g, yield 34.1%).
[0082] 1 HNMR (400MHz, CDCl3): δ5.11 (bs, 1H), 3.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com