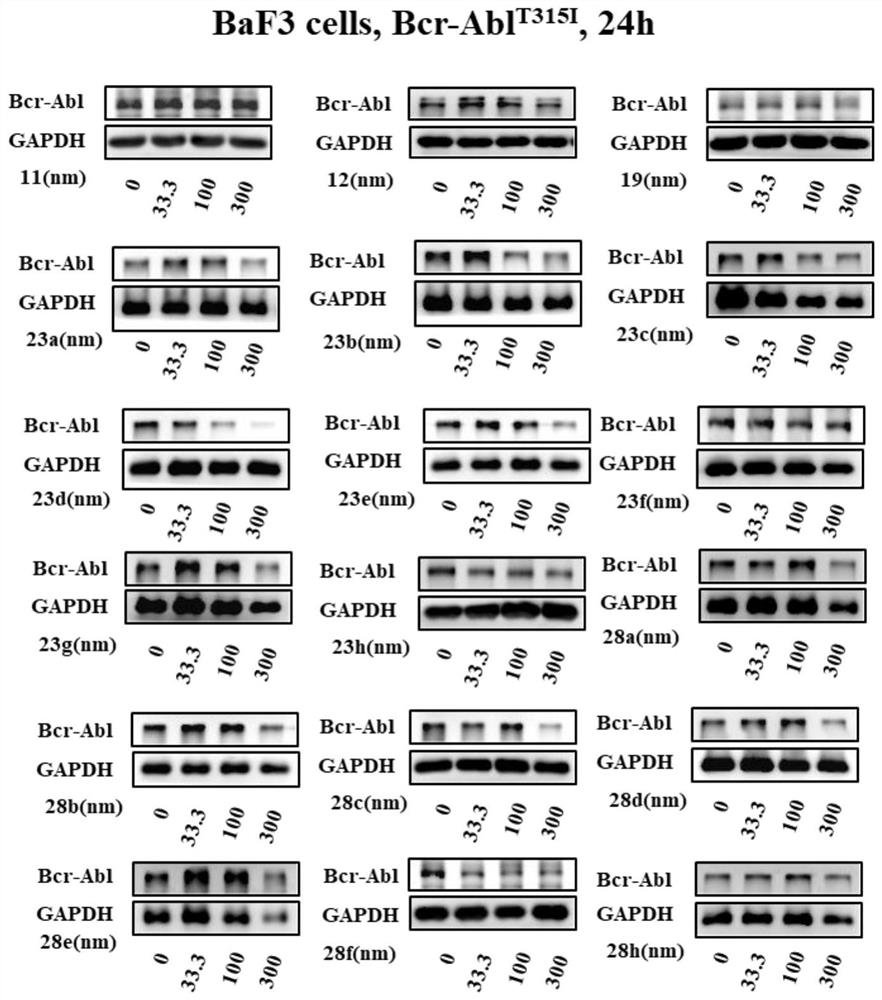
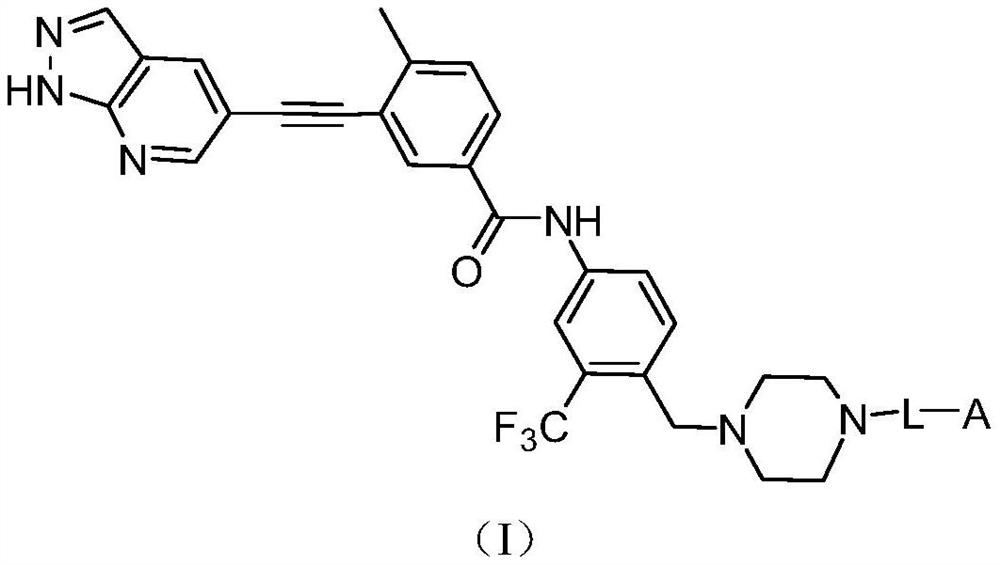
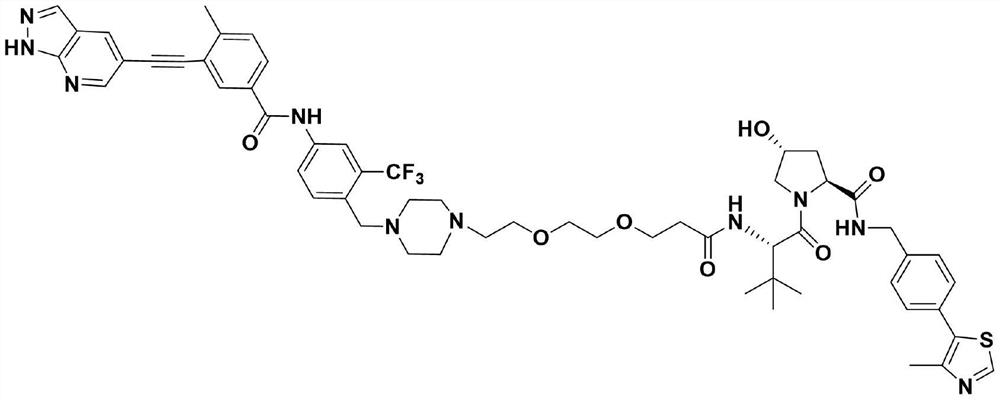
Protein degradation targeting chimera and application thereof
A protein degradation and chimera technology, applied in the field of chemical medicine, can solve problems such as affecting drug efficacy and poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0049] The reagents and raw materials used in the following examples are commercially available unless otherwise specified, and the corresponding Chinese meanings of the English abbreviations of the reagents are as follows:
[0050] NBS: N-bromosuccinimide; AIBN: azobisisobutyronitrile; DCM: dichloromethane; PE: petroleum ether; EA: ethyl acetate; TMSA: trimethylsilylacetylene; DMF: N , N-dimethylformamide; PMBCl: p-methoxybenzyl chloride; THF: tetrahydrofuran; TFA: trifluoroacetic acid; HATU: 2-(7-azabenzotriazole)-N,N, N',N'-tetramethylurea hexafluorophosphate; DIEA: N,N-diisopropylethylamine; TsCl: 4-toluenesulfonyl chloride.
Embodiment 1
[0051] Example 1: 3-((1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridin-5-yl)ethynyl)-4-methyl-N-( Preparation of 4-(piperazin-1-ylmethyl)-3-(trifluoromethyl)phenyl)benzamide (compound 8)
[0052]
[0053] Step a: Preparation of 1-(bromomethyl)-4-nitro-2-(trifluoromethyl)benzene (Compound 1)
[0054]
[0055] To a solution of 1-methyl-4-nitro-2-(trifluoromethyl)benzene (30 g, 146.3 mmol, 1 equiv) in 1,2-dichloroethane was added NBS (31.2 g, 175.5 mmol, 1.2 equiv ) and AIBN (2.4 g, 14.63 mmol, 0.1 equiv). The reaction mixture was heated at 90 °C overnight. After cooling, water was added to the mixture, the organic layer was separated, and the aqueous layer was extracted with DCM. The combined organic layers were washed with saturated brine and dried over anhydrous sodium sulfate. After removal of the solvent, the residue was purified by flash chromatography on silica gel (PE:EA=50:1) to give a pale yellow oil (6.1 g, 68%). 1 H NMR (400MHz, DMSO-d 6 )δ8.54(dd, J=8.5,2.5H...
Embodiment 2
[0077] Embodiment 2: the preparation of compound 11 and 12
[0078]
[0079] Step i: tert-butyl 3-(2-(2-(4-(4-(3-((1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridine Base-5-yl)ethynyl)-4-methylbenzamido)-2-(trifluoromethyl)benzyl)piperazin-1-yl)ethoxy)ethoxy)propionate ( Compound 9) Preparation
[0080]
[0081] To compound 8 (400mg, 0.62mmol, 1equiv) and 3-(2-(2-(tosyloxy)ethoxy)ethoxy) propanoic acid tert-butyl ester (316mg, 0.82mmol, 1.3equiv) To a solution of acetonitrile (30ml) was added anhydrous potassium carbonate (173mg, 1.25mmol, 2equiv), and the mixture was magnetically stirred at reflux temperature for 10 hours. After the reaction was finished, filter with suction and wash with CH 2 Cl 2 (2×25 mL), the filtrate was evaporated in vacuo and the residue was distilled under reduced pressure, the residue was purified by flash column chromatography on silica gel (DCM:CH 3 OH=15:1) afforded the product as a white solid (270 mg, 51%). 1 H NMR (400MHz, Chloroform-d)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com