Bcr-Abl amphiploid inhibitor, preparation method and application thereof
A solvent and compound technology, applied in the field of Bcr-Abl diploid inhibitors, can solve the problems of decreased affinity between imatinib and Abl kinase, decreased affinity, and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
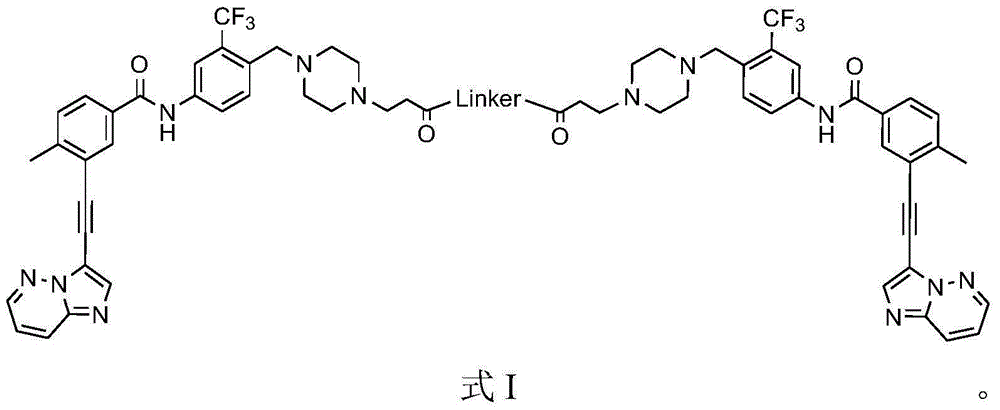
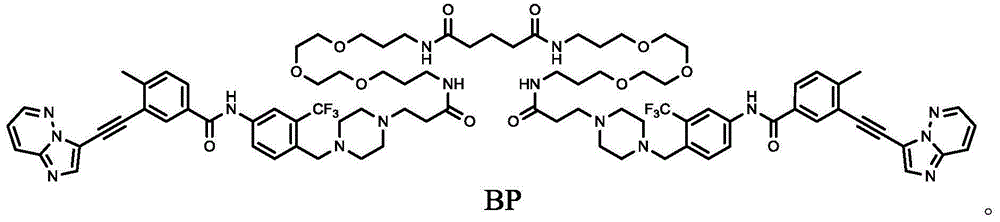
[0069] Example 1 Preparation of the compound BP of the present invention
[0070] The synthetic route is as follows:
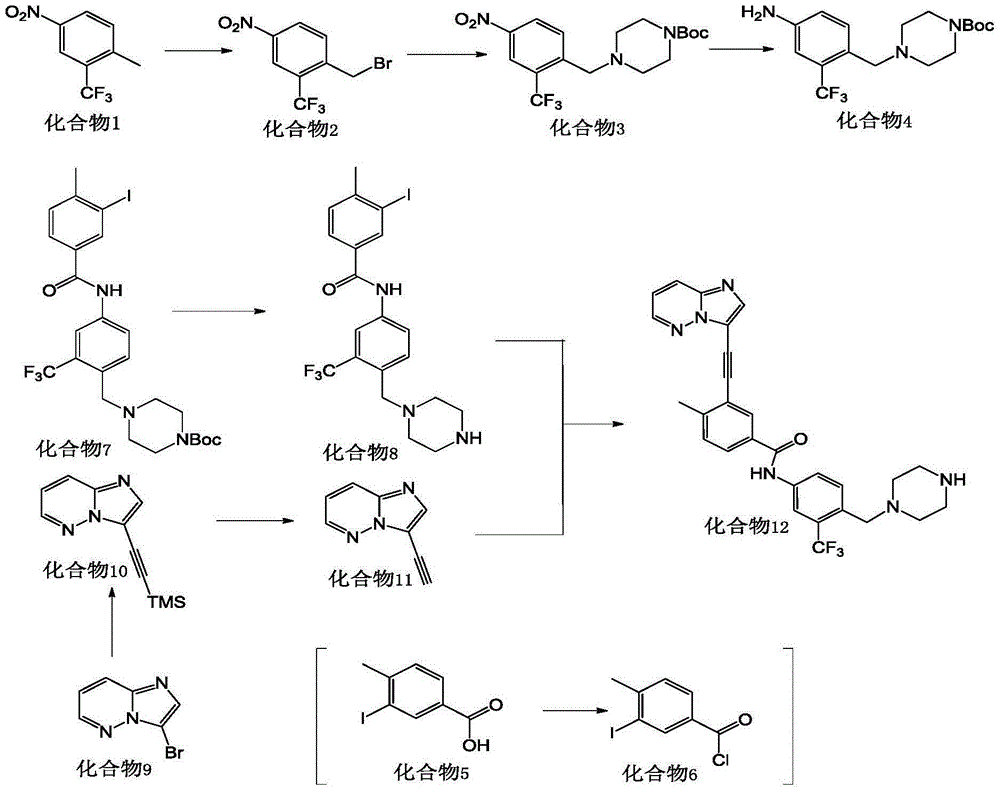
[0071] a. Preparation of compound 12:
[0072]
[0073] b. Preparation of compound BP:
[0074]
[0075] Preparation of compound 4
[0076] 2-Methyl-5-nitrotrifluorotoluene (compound 1) (30g, 0.15mol) was added to carbon tetrachloride (200ml), under nitrogen protection, N-bromosuccinimide (28.8 g, 0.16 mol), AIBN (2.5 g, 0.01 mol), stir electromagnetically, heat up to reflux for 24 h, stop the reaction, cool to room temperature, filter the reaction solution, wash the solid with ethyl acetate, combine the ethyl acetate phases, and use Wash with saturated aqueous sodium bicarbonate solution, then with saturated aqueous sodium chloride solution (10 ml×2), dry over anhydrous sodium sulfate, evaporate the solvent to dryness to obtain a yellow oil, which is compound 2, which is directly used in the next step without purification.
[0077] Preparation of co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com