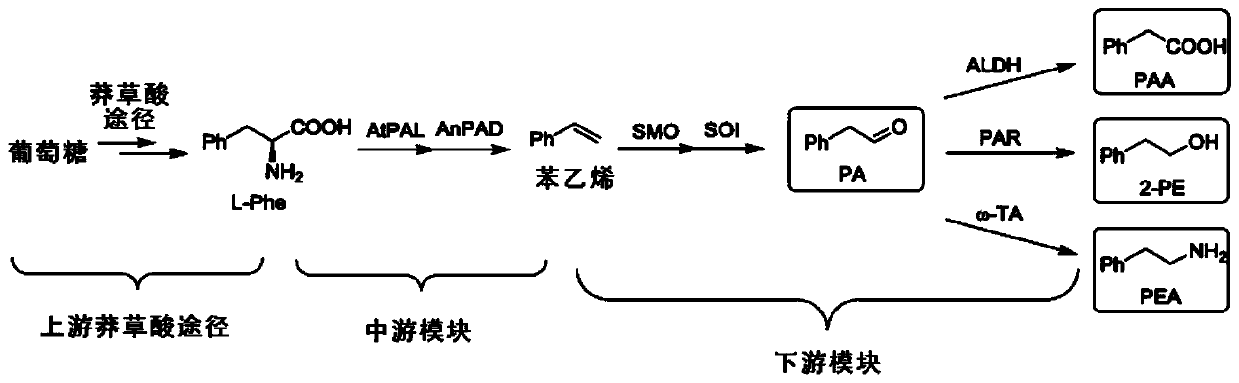
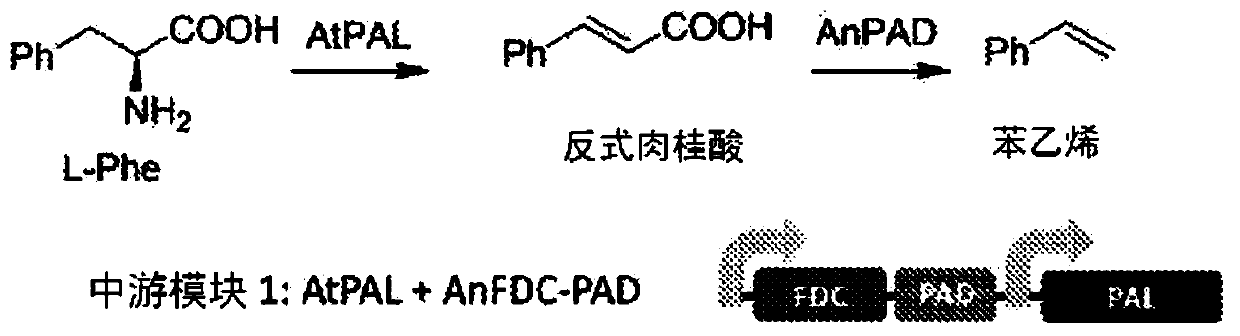
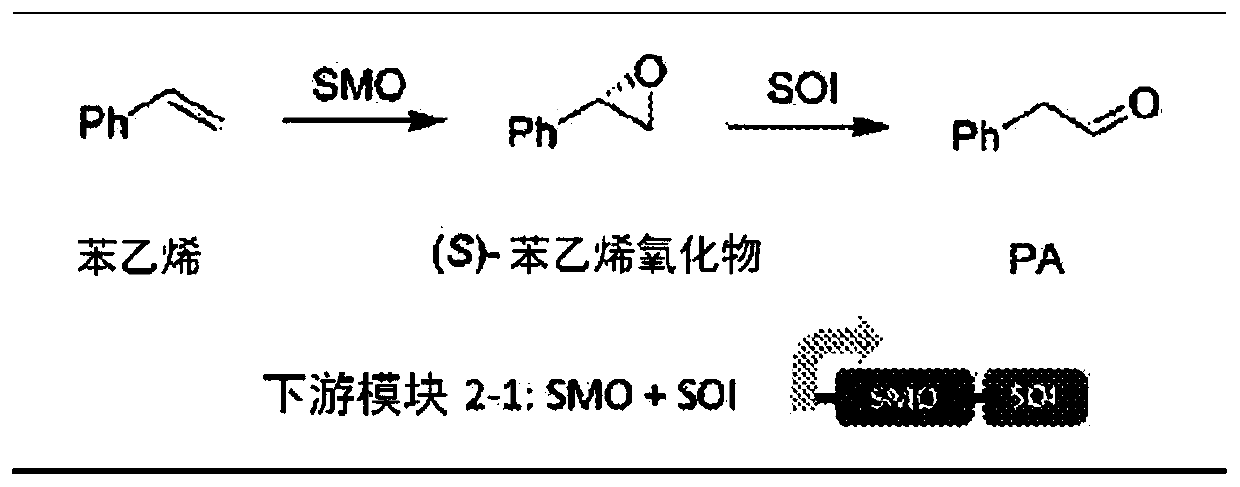
Bioproduction of phenethyl alcohol, aldehyde, acid, amine, and related compounds
A technology of phenylethanol and phenylacetaldehyde, applied in biochemical equipment and methods, oxidoreductase, lyase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Example 1. Genetic engineering of Escherichia coli containing module 2-1 and expressing SMO and SOI
[0163] The styC gene encoding the SOI (SEQ ID NO: 9) from Pseudomonas sp. VLB120 was first synthesized and codon-optimized for E. coli according to the published sequence. Primers StyC-KpnI-RBS-F (CGGGTACCTAAGGAGATATATAATGTTACACGCGTTTGAACG TA AAATG; SEQ ID NO: 29) and StyC-HindIII-XhoI-R (ACTGCTCGAGAAGCTTACTCGGCTGCCGCGTGTGGAACGGC TTTACG; SEQ ID NO: 30) and Phusion DNA polymerase (available from Thermo) were then used Amplify it. The PCR product was double-digested with KpnI and XhoI, and then ligated with the pRSF-SMO plasmid after the same digestion with T4 DNA ligase [Wu, S., Chen, Y., et al., ACS Catal.4:409- 420 (2014)]. The ligation product was transformed (heat shock) into E. coli T7 expression competent cells (available from New England Biolabs) to yield pRSF-SMO-SOI. This module 2-1 was subcloned into the other three vectors by the following procedure. The m...
Embodiment 2
[0164] Example 2. Genetic Engineering of Escherichia coli Containing Module 2-2 and Expressing SMO, SOI and PAR
[0165] First, the pad gene encoding ADH (alcohol dehydrogenase; SEQ ID NO: 11) from Saccharomyces cerevisiae was synthesized, and the codon optimization for Escherichia coli was performed according to the published sequence. It was then processed using primers PAR-HindIII-RBS-F (ACTGAAGCTTTAAGGAGATATATAATGAGCGTGACCGCGAAA ACCGTG; SEQ ID NO: 32) and PAR-XhoI-R (ACTGCTCGAGTCACATGCTTGAACTCCCG CCGAAA; SEQ ID NO: 33) and Phusion DNA polymerase (available from Thermo) Amplify. The PCR product was double digested with HindIII and XhoI, and then ligated with the same digested pRSF-SMO-SOI plasmid with T4 DNA ligase (see Example 1). The ligation product was transformed (heat shock) into E. coli T7 expression competent cells (available from New England Biolabs) to yield pRSF-SMO-SOI-PAR. This module 2-2 was subcloned into the other three vectors by the following procedure. ...
Embodiment 3
[0166] Example 3. Production of 2-PE from Sty via cascade biocatalysis by using E. coli containing module 2-2 and expressing SMO, SOI and PAR
[0167] The recombinant Escherichia coli (StyABC-PAR) containing the plasmid pRSF-SMO-SOI-PAR was grown at 37°C in 1 mL of LB medium containing 50 mg / L kanamycin, and then inoculated into 50 mL containing glucose (20 g / L L), yeast extract (6g / L) and 50mg / L kanamycin in the M9 medium. When OD 600 When 0.6 was reached, 0.5 mM IPTG was added to induce expression of the enzyme. Cells continued to grow and express protein for 12 hours at 22°C, after which they were harvested by centrifugation (4000 g, 10 min). Cells were resuspended in 200 mM KPB buffer (pH=8.0) with 2% glucose (for cofactor regeneration) to 10 gcdw / L. To 2 mL of the aqueous system, 2 mL of n-hexadecane containing 60 mM Sty was added to the reaction system to form a second phase. The reaction was carried out in a 100 mL flask at 30 °C and 300 rpm for 8 hours. During the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com