Subcutaneous dosing of Anti-cd38 antibodies
A technology for subcutaneous administration of antibodies, applied in the direction of antibodies, anti-tumor drugs, antibody medical components, etc., can solve problems such as severe infusion-related reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0339] Example 1: Model-Based Characterization of Anti-CD38 Antibodies in Cynomolgus Monkeys
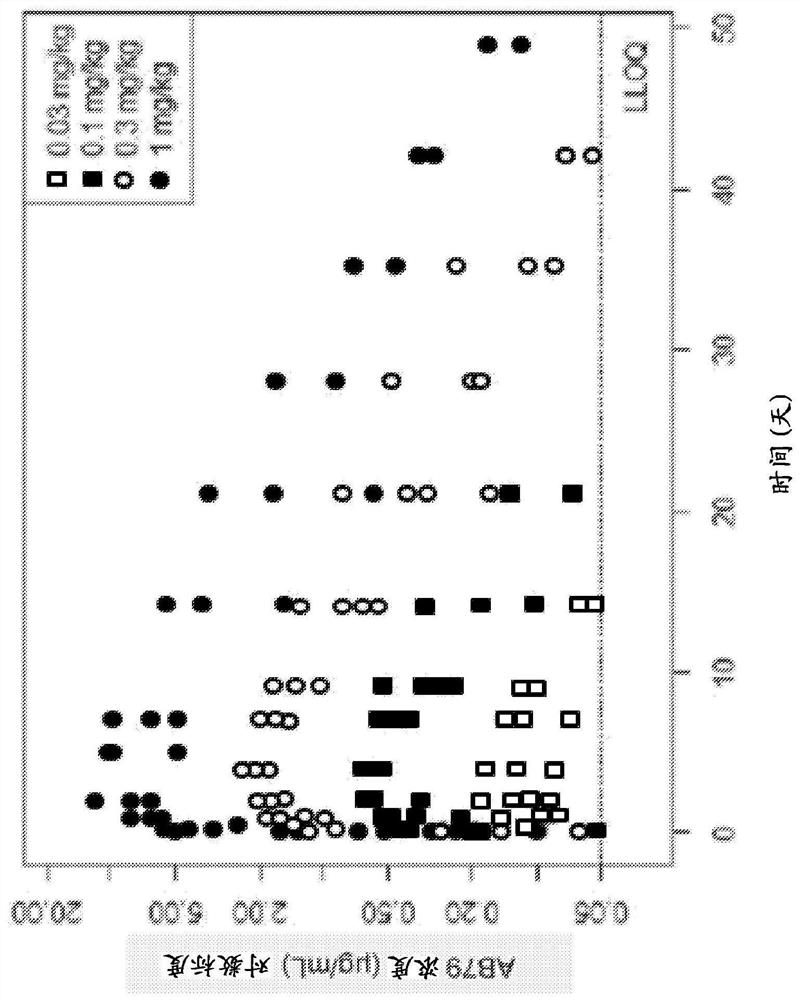
[0340] The anti-CD38 antibody AB79 binds to cynomolgus monkey (cyno) CD38, thereby rendering it incompatible with Daratumumab (Darzalex TM ) (a cytolytic CD38 monoclonal antibody recently approved for the treatment of multiple myeloma). This unique capability supports the use of cynomolgus monkeys in preclinical studies to characterize AB79 pharmacokinetics (PK), pharmacodynamics (PD), and safety. To this end, assays were developed to measure drug concentration, immunogenicity and quantify T cells, B cells and NK lymphocytes in the blood of cynomolgus monkeys. These parameters were evaluated in eight pharmacological and toxicological preclinical studies. Among the cell populations tested, CD38 expression was highest on NK cells; therefore, the drug effect on NK cells was assumed to be closest to that on the target cells considered, plasmablasts, plasma cells and other activated lym...
Embodiment 2
[0422] Example 2: AB79 depletes CD38+ cells
[0423] CD38 is a cADPR hydrolase expressed on human fibroblasts, plasma cells, NK cells and activated T and B cells, but not on mature platelets or erythrocytes based on AB79 binding. In patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), plasma cells and activated B and T cells may be important contributors to the disease. with targeting CD20 and not directly depleting CD20 低 / 阴性 Unlike other B-cell selective therapies for plasmablasts, CD38 is expressed at high levels on plasmablasts and plasma cells, making these cells a direct target of AB79. In vitro studies in human blood cells and cell lines showed that binding of AB79 to CD38 did not result in PBMC cytokine activation, thus demonstrating that AB79 is not an agonist, as discussed below. In contrast, AB79 mediated cell depletion of human B lineage cell lines via ADCC and CDC, and in most cases, cell lines with increased CD38 expression were more p...
Embodiment 3
[0446] Example 3: For the study of the safety, tolerability, efficacy, Phase 1 / 2a study of pharmacokinetics and immunogenicity
[0447] The purpose of this study is to evaluate safety, tolerability, pharmacokinetics (PK), immunogenicity, dose-limiting toxicity (DLT) and maximum tolerated dose (MTD) in Phase 1 of the study / recommended Phase 2 dose (RP2D), and provides a preliminary evaluation of the clinical activity of AB79 monotherapy in subjects with relapsed and / or refractory multiple myeloma (RRMM). The study included patients with RRMM previously treated with at least proteasome inhibitors (PIs), immunomodulatory drugs (IMids), alkylating agents and steroids. Patients should have refractory disease or be intolerant to at least 1 PI and at least 1 IMiD, and they should have received 3 or more prior therapies if one of those therapies included a combination of PI and IMiD , or have received at least 2 prior therapies. During the phase 1b dose escalation portion, prior ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com