Novel inhibitors of guanosine monophosphate synthetase as therapeutic agents
A compound, free technology, applied in the field of novel guanosine monophosphate synthase inhibitors, which can solve the problems of lack of effective and specificity, limited utilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0097] Development of new monophosphate osteoside synthase inhibitors
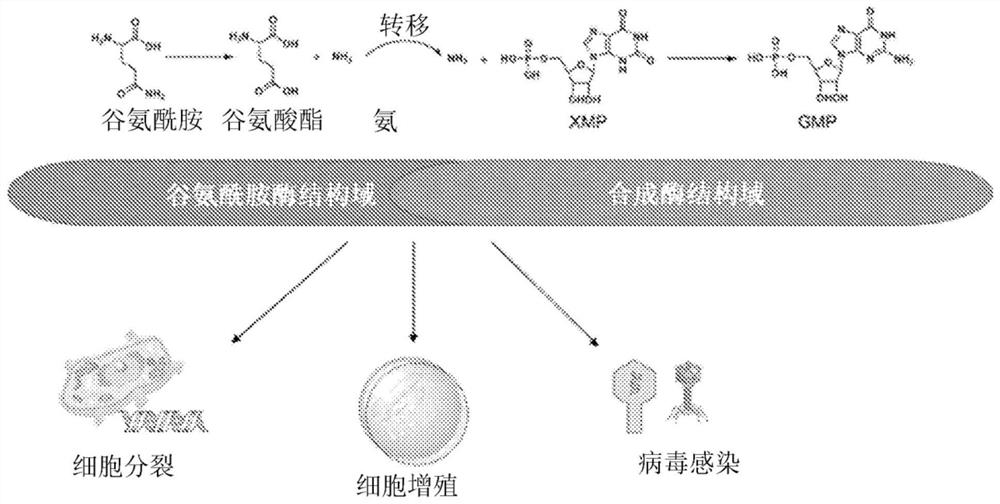
[0098] Single phosphate chinelide enzyme (GMPS) is a dual-domain enzyme that produces ammonia, which is transferred to the synthetic domain to convert XMP to GMP (nucleotide precursor of RNA). . Therefore, and figure 1 The GMPS is shown to play an important role in cell division and proliferation and viral infection.
[0099] A series of new compounds have been developed in the lack of effective and specific GMPS inhibitors, and their ability to induce GMPS enzyme activity inhibition is developed.
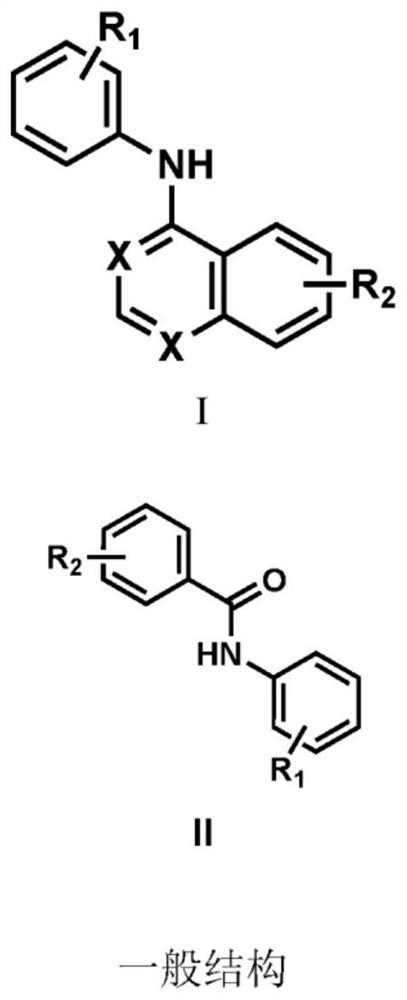
[0100] like figure 2 The developed compounds shown have the structure as described in two brackets (I) and (II). In general structures (I) and (II), X is N or CH, R 1 Is (alkyl, acyl, alkoxy, halo, amino, amide, alkenyl, alkynyl), and R 2 It is (alkyl, acyl, alkoxy, halo, amino, amide, alkenyl, alkynyl).
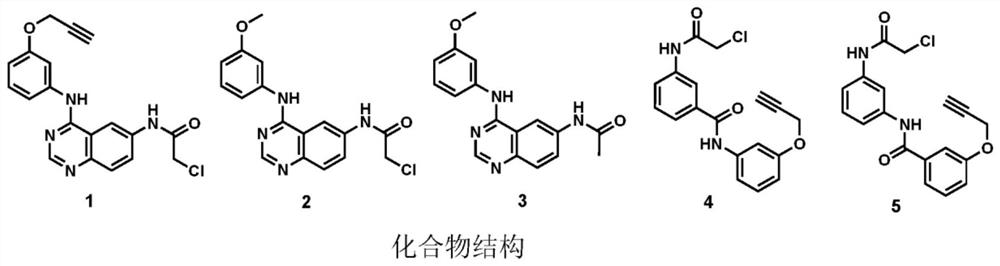
[0101] like image 3 The pre-reporting conditions and modified composite structures (1-5) based on two bracket...
example 2
[0103] Evaluation of efficacy of GMPS inhibitor
[0104] By monitoring the UV absorbance at 290 nm and the five compounds were tested in the enzymatic determination by determining IC50, the efficacy of recombinant GMPs was tested. Summarized the IC obtained in Table 3 50 value. Three compounds (1, 2 and 4) in namolar IC 50 Value inhibits GMPS while discovering that the other two (3 and 5) is low. Structure - Activity Relationship (SAR) Indicates that the chloroacetamide group is essential for effective combination with GMPS.
[0105] Compound IC 50 (nm)
[0106] Table 3. Determination of compounds in recombinant human GMPS IC 50 value.
example 3
[0108] Assessment of covalent combination of compound 1 and GMPS in living cells
[0109] In order to assess the binding ability of GMPs in the alternating cell, the most effective inhibitor (i.e., compound 1) is evaluated covalently bonded to GMPs in the live cells.
[0110] HEK293 cells were treated with various concentrations for 20 minutes. Cells were washed with DPBS (dubelco phosphate buffer), centrifuged, and then cleaved using NP40-based buffer. The resulting cell lysate and TAMRA laminate subjected to Cu-catalyzed azide-alkyne ring addition reaction (CuAAc), and then analyzed by SDS PAGE and visualized TAMRA fluorescence. like Figure 4A The display is observed at approximately 75 kDa to a protruding strip that matches the size of the GMP. This result indicates that the compound 1 is covalently bonded to the GMPS, as the observed it is expected to be expected for the importance of the parental bond that binds to the target. Further, the dose reaction indicates that the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com