Methods and compositions for increasing protein expression and/or treating a haploinsufficiency disorder
A dose-and-deficient technology for use in biochemical devices and methods, drug combinations, gene therapy, etc., to address problems such as language and motor dysfunction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] Preparation of viral vectors
[0142] Methods of preparing viral vectors are known in the art. Generally, known viruses are prepared in suitable host cell lines using conventional techniques, including culturing transfected or infected host cells under suitable conditions so as to produce infectious virus particles. Nucleic acids encoding viral genes and / or tRNAs can be integrated into plasmids and introduced into host cells by conventional transfection or transformation techniques. Examples of host cells for producing known viruses include human cell lines such as HeLa, Hela-S3, HEK293, 911, A549, HER96 or PER-C6 cells. Specific preparation and purification conditions will vary depending on the virus and production system used.
[0143] In certain embodiments, producer cells can be administered directly to a subject, however, in other embodiments, following production, infectious viral particles are recovered from culture and optionally purified. Typical purificatio...
Embodiment
[0173] The following examples are illustrative only and are not intended to limit the scope or content of the invention in any way.
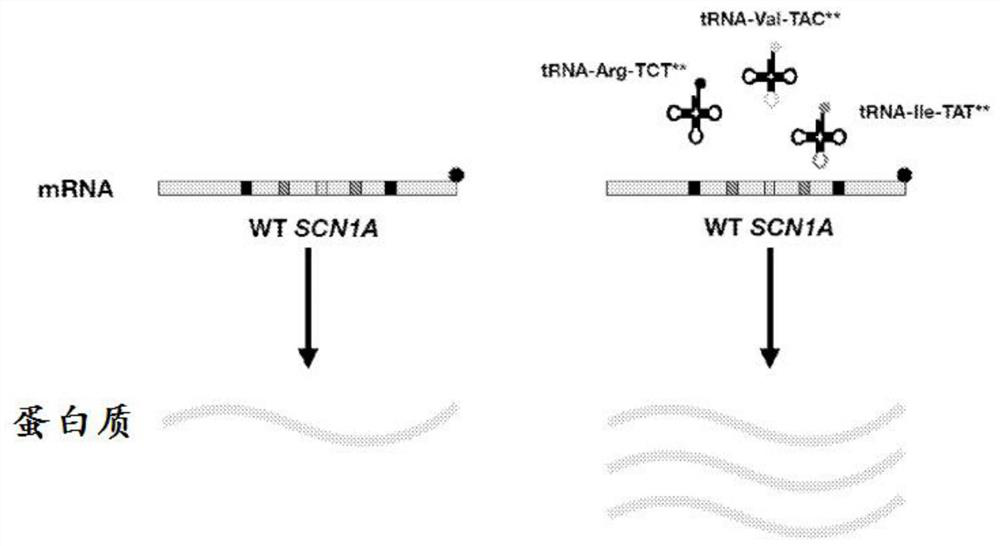
[0174] Example 1 - tRNA expression that enhances expression of wild-type SCN1A transcript
[0175] In this example, the least desirable and most overexpressed codons in SCN1A were determined. Expression of cognate tRNAs to these codons could enhance SCN1A expression and treat Dravet syndrome.
[0176] First, the percent usage of each codon in the SCN1A gene was determined. Subsequently, the optimality of each codon in the SCN1A gene was determined using the tRNA fitness index (tAI). tAI is an estimate of translation efficiency for each codon, which takes into account estimates of tRNA cellular concentration (based on tRNA gene copy number) and decoding efficiency, with higher tAI values indicating more optimal codons. For example, tAI is described in dos Reis et al., (2004) NUCLEIC ACIDS RES. 32(17):5036-5044 and Mahlab et al., (2014) PLoS ...
Embodiment 2
[0182] Example 2 - tRNA expression that enhances expression of wild-type SCN1A transcript
[0183] In this example, the ability of ectopic delivery of selection transfer RNAs (tRNAs) to enhance wild-type SCN1A expression in cultured cells was assessed.
[0184] ATA (Ile), GTA (Val) and AGA (Arg) were chosen as exemplary non-optimal codons in the SCN1A gene. Described in SEQ ID NO: 1 coding ATA tRNA (tRNA ATA ), described in SEQ ID NO: 2 encoding GTA tRNA (tRNA GTA ) of the nucleotide sequence described in SEQ ID NO: 3 encoding AGA tRNA (tRNA AGA ) nucleotide sequence.
[0185] The SCN1A open reading frame was amplified from human brain RNA (Clontech) by RT-PCR using an upstream primer containing a KpnI site, a downstream primer containing a NotI site. The SCN1A open reading frame was then cloned between the promoter and the SV40 poly(A) site of pTRE-Tight (Clontech) using KpnI and NotI sites.
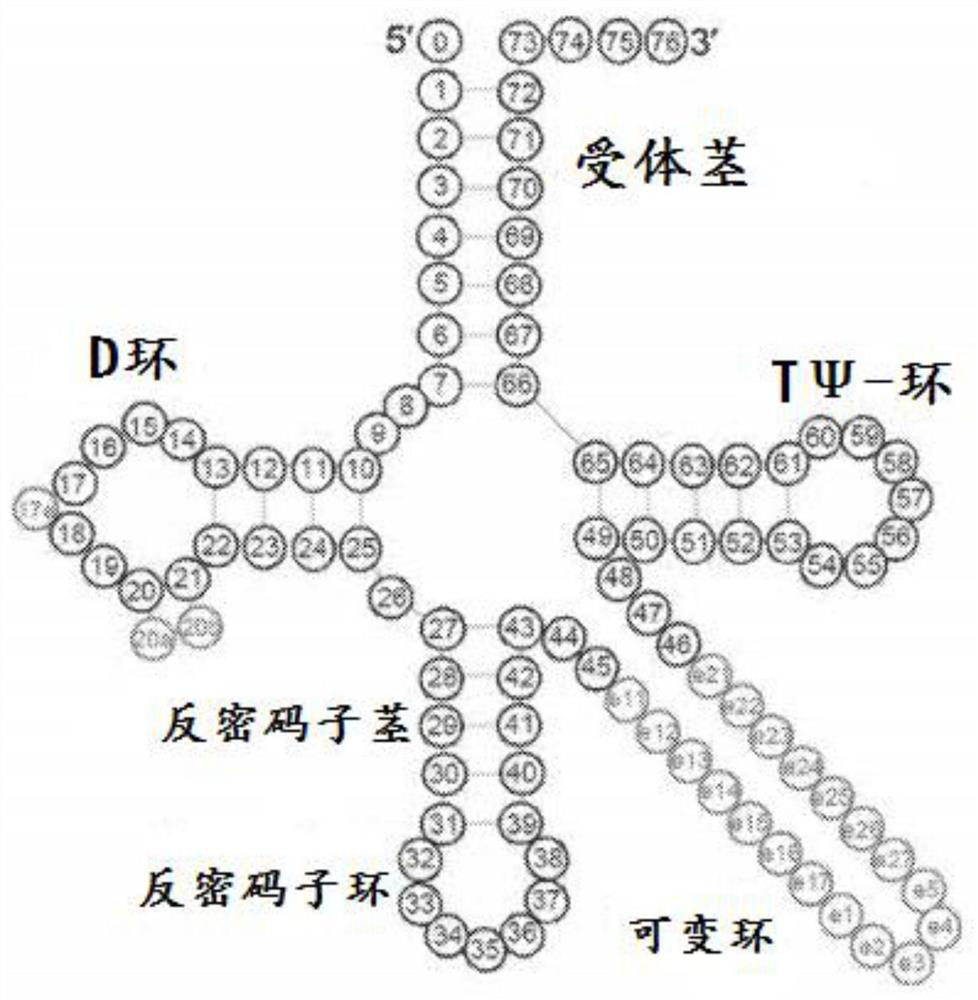
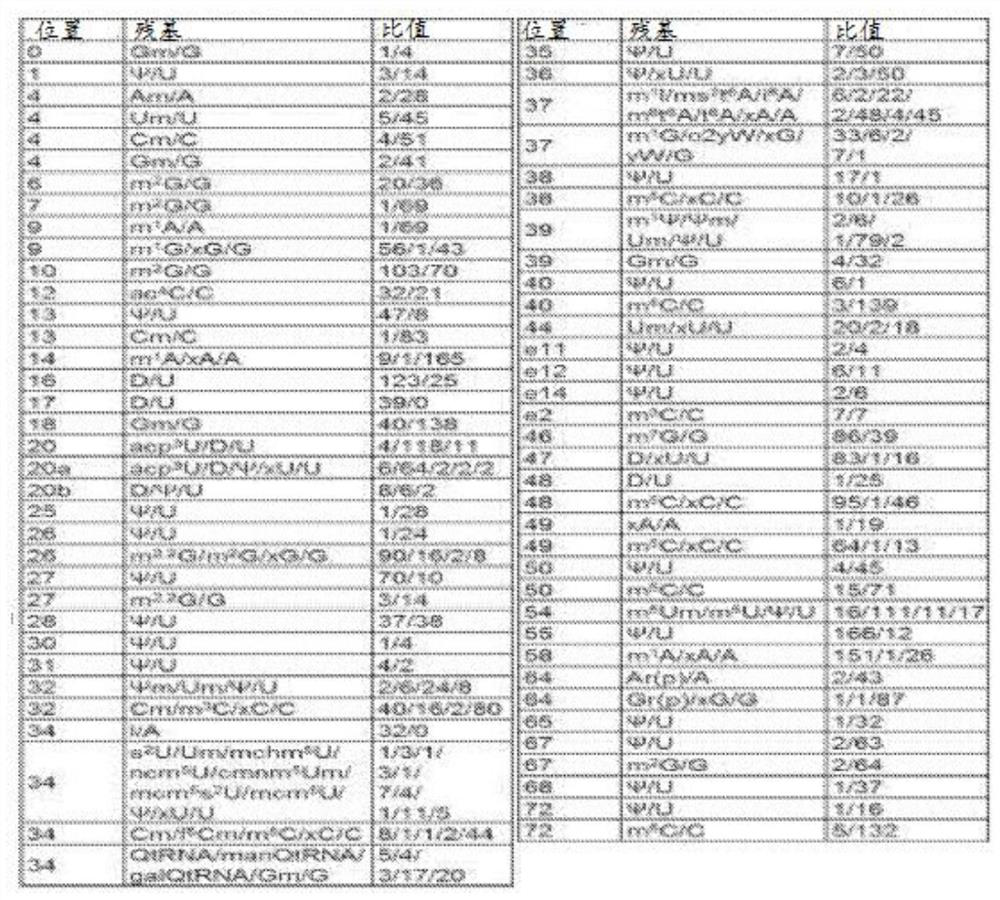
[0186] tRNA ATA , tRNA GTA and tRNA AGA The gene sequence (including 200 ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com