Recombinant adeno-associated virus for treatment of grn-associated adult-onset neurodegeneration
A technology of viruses and uses, applied to nervous system diseases, single-stranded DNA viruses, viruses, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0140]
[0141]
[0142]
[0143]
[0144]
example 1
[0145] Example 1: Materials and methods
[0146] carrier
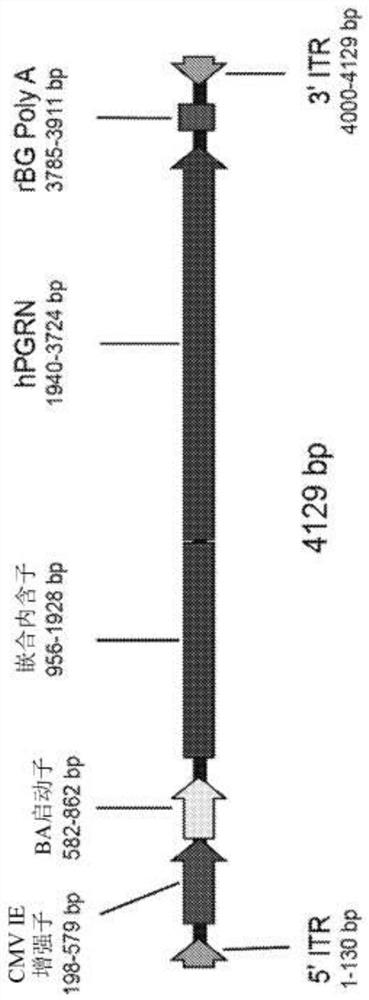
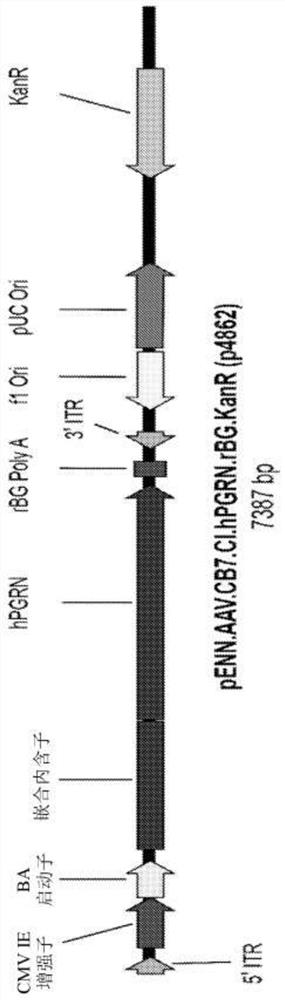
[0147] Cloning of the engineered human PGRN cDNA into an expression construct containing the chicken β-actin promoter with the cytomegalovirus early enhancer, chimeric intron, and rabbit β-globin polyadenylation sequence ( figure 1 ). The second engineered human PGRN cDNA was cloned into an expression construct containing the human ubiquitin C promoter. The expression construct was flanked by AAV2 inverted terminal repeats. Adeno-associated virus serotypes 1, 5 and human 68 (AAVhu68) were generated from this construct by triple transfection of HEK293 cells and purification with iodixanol as previously described (Lock M et al., Human Gene Therapy 2010; 21(10):1259-71).
[0148] animal procedure
[0149] All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. A breeding pair of GRN knockout mice (stock no. 013175) was purchased from The Jackson labo...
example 2
[0161] Example 2: AAV-mediated delivery of human GRN transgenes in murine disease models
[0162] Recombinant AAV vectors with expression under the control of the CB7 promoter and chimeric intron (CB7.CI.hPGRN.rBG) were produced using the published triple transfection technique as described, for example, in WO 2018 / 160582 AAVhu68 capsid of human PGRN (SEQ ID NO:3).
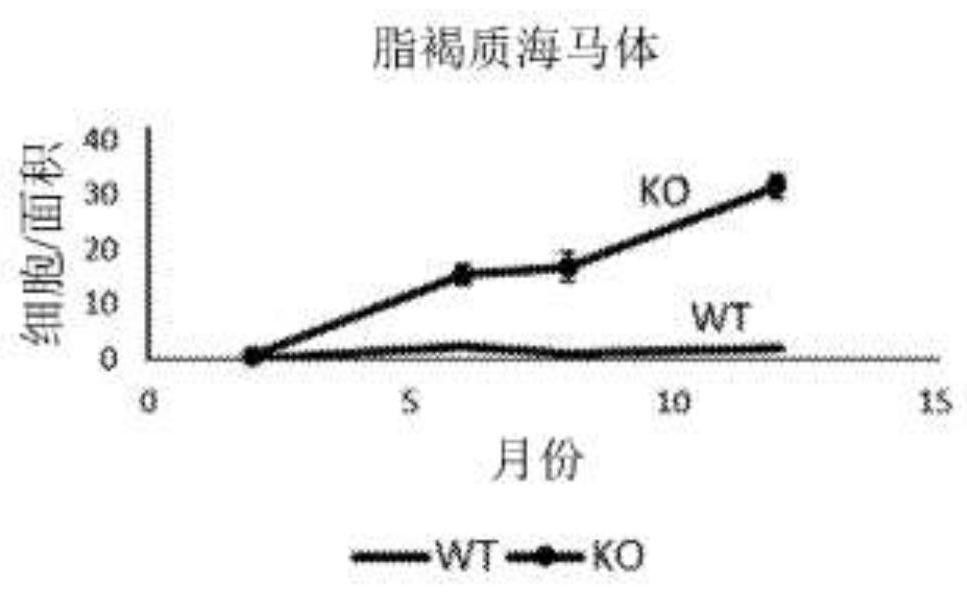
[0163] Evaluation of AAV-mediated delivery of the human GRN transgene in a GRN knockout mouse model. Mice heterozygous for the GRN mutation (GRN + / - ) did not exhibit the pathological features of GRN-associated neurodegenerative diseases, possibly because the mouse lifespan did not allow for the sequelae of GRN haplotype insufficiency that were first manifested in humans decades later. In contrast, GRN - / - Intact PGRN deficiency in mice recapitulates several early features of human GRN haplotype deficiency, such as impaired lysosomal function, accumulation of autofluorescent lysosomal storage material (lipofusc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com